NYXOID 1,8 MG SOLUCION PARA PULVERIZACION NASAL EN ENVASE UNIDOSIS


Cómo usar NYXOID 1,8 MG SOLUCION PARA PULVERIZACION NASAL EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Nyxoid 1,8 mg solución para pulverización nasal en envase unidosis
naloxona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Nyxoid y para qué se utiliza
- Qué necesita saber antes de empezar a usar Nyxoid
- Cómo usar Nyxoid
- Posibles efectos adversos
- Conservación de Nyxoid
- Contenido del envase e información adicional
1. Qué es Nyxoid y para qué se utiliza
Este medicamento contiene el principio activo naloxona. La naloxona revierte temporalmente los efectos de opioides como la heroína, metadona, fentanilo, oxicodona, buprenorfina y morfina.
Nyxoid es una solución para pulverización que se utiliza para el tratamiento de urgencia de la sobredosis, o posible sobredosis, por opioides en adultos y adolescentes de 14 años de edad en adelante. Los signos de sobredosis incluyen:
- problemas para respirar
- somnolencia extrema
- no responder a un ruido fuerte o al tacto
Si usted está en riesgo de sobredosis por opioides, debe llevar siempre consigo Nyxoid.Nyxoid funciona solo temporalmente para revertir los efectos de los opioides mientras espera atención médica de urgencia. No es un sustituto de la atención médica de urgencia. Nyxoid debe ser usado por personas con la preparación adecuada.
Informe siempre a sus amigos y familiares de que lleva Nyxoid con usted.
2. Qué necesita saber antes de empezar a usar Nyxoid
No use Nyxoid
Si es alérgico a naloxona o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Nyxoid solo se le proporcionará después de que usted o su cuidador hayan recibido formación sobre cómo usarlo.
Se debe administrar inmediatamente y no sustituye a la atención médica de urgencia.
- Debe llamar al servicio de urgencias si se sospecha una sobredosis por opioides.
Los signos y síntomas de una sobredosis por opioides pueden volver a aparecer después de administrar este pulverizador nasal. Si esto sucede, se pueden administrar otras dosis después de 2 a 3 minutos, utilizando un nuevo pulverizador nasal. Después de recibir este medicamento, el paciente debe permanecer bajo una estrecha supervisión hasta que llegue la ayuda de urgencia.
Problemas médicos a los que se debe prestar atención
- Si tiene dependencia física a los opioides o ha recibido dosis altas de opioides (por ejemplo, heroína, metadona, fentanilo, oxicodona, buprenorfina o morfina). Puede experimentar fuertes síntomas de abstinencia con este medicamento (ver más adelante en la sección 4 de este prospecto, bajo “Problemas médicos a los que se debe prestar atención”).
- Si toma opioides para controlar el dolor. El dolor puede aumentar cuando reciba Nyxoid.
- Si utiliza buprenorfina. Puede que Nyxoid no revierta completamente los problemas respiratorios.
Informe a su médicosi tiene daños en el interior de la nariz, ya que esto podría afectar al funcionamiento de Nyxoid.
Niños y adolescentes
Nyxoid no se debe usar en niños ni en adolescentes menores de 14 años.
Administración de Nyxoid en madres próximas al parto
Informe a su médico o a su matronasi ha usado Nyxoidpróximo al partoo durante el mismo.
Su bebé podría sufrir un síndrome de abstinencia a opioides repentino, que podría poner en peligro su vida si no se trata.
Durante las 24 horas posteriores al nacimiento del bebé, esté atenta a los siguientes síntomas en su bebé:
- convulsiones (ataques)
- más llanto del habitual
- aumento de los reflejos
Otros medicamentos y Nyxoid
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de recibir un suministro de este medicamento. Si se le administra Nyxoid mientras está embarazada o en período de lactancia, su bebé debe permanecer bajo estrecha supervisión.
Conducción y uso de máquinas
Después de tomar este medicamento, no debe conducir, utilizar máquinas o participar en otras actividades que exijan un esfuerzo físico o mental durante al menos 24 horas, ya que el efecto de los opioides puede volver.
Nyxoid contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Nyxoid
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Se proporcionará formación sobre cómo usar Nyxoid antes de que se le suministre. A continuación proporcionamos una guía paso a paso.
Instrucciones para administrar Nyxoid solución para pulverización nasal
- Compruebe los síntomas y la respuesta.
- Compruebe si hay respuesta para ver si la persona está consciente.Puede gritar su nombre, agitarle los hombros suavemente, hablarle alto al oído, frotarle el esternón, pellizcarle la oreja o la base de una uña.
- Compruebe las vías respiratorias y la respiración.Libere la boca o la nariz de cualquier bloqueo. Compruebe la respiración durante 10 segundos. ¿Se mueve el pecho? ¿Puede oír sonidos de respiración? ¿Puede sentir la respiración en la mejilla?
- Compruebe los signos de sobredosis, como: sin respuesta al tacto o el sonido, respiración lenta e irregular o ausencia de respiración, ronquidos, jadeos o respiraciones rápidas, uñas o labios morados o azules, pupilas muy pequeñas.
- Si se sospecha que hay sobredosis, se debe administrar Nyxoid lo antes posible.
- Llame a una ambulancia.Nyxoid no es un sustituto de la atención médica de urgencia.
|
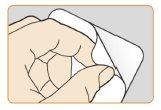
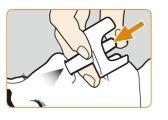
- Despeguela parte posterior del blíster por una esquina para sacar el pulverizador nasaldel envase. Coloque el pulverizador nasal en un lugar de fácil acceso.
|
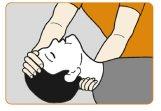
- Tumbe al paciente boca arriba. Sujete el cuello por la parte posterior permitiendo que la cabeza se incline hacia atrás. Despeje cualquier cosa que pueda bloquear la nariz.
|
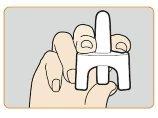
- Sujete la parte inferior del émbolo del pulverizador nasal con el dedo pulgar y coloque los dedos índice y corazón a ambos lados de la boquilla. No cebe ni pruebe el dispositivo antes de suusoya que contiene solo una dosis y no se puede volver a utilizar.
- Introduzca suavemente la boquilla del dispositivo en una de las fosas nasales. Presione confirmezael émbolo hasta que haga clicpara administrar la dosis. Retire la boquilla del pulverizador nasal de la fosa nasal después de administrar la dosis.
- Coloque al paciente en posición de recuperaciónsobre su costado, con la boca
abierta orientada hacia el suelo y permanezca con el paciente hasta que lleguen los servicios de urgencias. Observe si hay alguna mejoría en la respiración del paciente, en su estado de alerta y en la respuesta al ruido o al tacto.
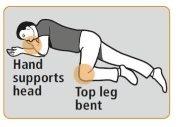
La mano | ||
sujeta la | ||
cabeza | ||
Parte superior | ||
de la pierna doblada | ||
- Si el paciente no mejoraen 2-3 minutos, se le puede administrar una segunda dosis. Tenga en cuenta que incluso si se despierta, puede volverse a quedar inconsciente y dejar de respirar. Si esto sucede, se puede administrar una segunda dosis inmediatamente. Administre Nyxoid en la otra fosa nasal utilizando un pulverizador nasal Nyxoid nuevo. Esto se puede hacer mientrasel paciente se encuentra en la posición de recuperación.
- Si el paciente no responde a dos dosis, se pueden administrar otras dosis (si están disponibles). Quédese con el paciente y continúe observando si hay alguna mejora hasta que lleguen los servicios de urgencia que administrarán el tratamiento posterior.
En pacientes que estén inconscientes y que no respiren normalmente, cuando sea posible, se deben aplicar medidas de apoyo adicionales para salvarles la vida.
Para obtener más información o vídeos, escanee el código QR o visite www.nyxoid.com
Si tiene más preguntas sobre el uso de este medicamento, consulte con su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Con este medicamento pueden tener lugar los efectos adversos que siguen.
Problemas médicos a los que se debe prestar atención
Nyxoid puede causar síntomas de abstinencia agudossi el paciente tiene dependencia de los opioides. Los síntomas pueden incluir: El síndrome de abstinencia de la droga incluye inquietud, irritabilidad, hiperestesia (aumento de la sensibilidad de la piel), náuseas (sensación de malestar), vómitos (estar enfermo), dolor gastrointestinal (calambres de estómago), espasmos musculares (una tensión repentina de los músculos, dolores corporales), disforia (estado de ánimo desagradable o incómodo), insomnio (dificultad para dormir), ansiedad, hiperhidrosis (sudoración excesiva), piloerección (piel de gallina, escalofríos o temblores), taquicardia (ritmo cardíaco acelerado), aumento de la presión arterial, bostezos, pirexia (fiebre). También pueden observarse cambios de comportamiento, como conducta violenta, nerviosismo y excitación.
Los síntomas de abstinencia agudos ocurren de forma poco frecuente (pueden afectar hasta a 1 de cada
- personas).
Informe a su médicosi experimenta alguno de estos síntomas.
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- Sensación de enfermedad (náuseas)
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Mareo, dolor de cabeza
- Frecuencia cardíaca rápida
- Presión arterial elevada, presión arterial baja
- Vómitos
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- Temblor
- Frecuencia cardíaca lenta
- Sudoración
- Frecuencia cardíaca irregular
- Diarrea
- Sequedad de boca
- Respiración rápida
Muy raros: pueden afectar hasta 1 de cada 10.000 personas
- Reacciones alérgicas, como hinchazón de la cara, la boca, los labios o la garganta, shock alérgico
- Latidos cardíacos irregulares y potencialmente mortales, infarto de miocardio
- Acumulación de líquido en los pulmones
- Problemas cutáneos como picazón, sarpullido, enrojecimiento, hinchazón o descamación intensa de la piel
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nyxoid
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, el blíster y la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
No congelar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nyxoid
- El principio activo es naloxona. Cada pulverizador nasal contiene 1,8 mg de naloxona (como hidrocloruro dihidrato).
- Los demás componentes son citrato trisódico dihidratado (E331), cloruro de sodio, ácido clorhídrico (E507), hidróxido de sodio (E524) y agua purificada (Ver “Nyxoid contiene sodio” en sección 2).
Aspecto del producto y contenido del envase
Este medicamento contiene naloxona en 0,1 ml de una solución transparente, de incolora a amarillo pálido en un pulverizador nasal precargado, solución en un envase unidosis (pulverización nasal, solución)
Nyxoid se acondiciona en una caja de cartón que contiene 2 pulverizadores nasales cerrados en blísteres individuales. Cada pulverizador nasal contiene una única dosis de naloxona.
Titular de la autorización de comercialización
Mundipharma Corporation (Ireland) Limited
United Drug House Magna Drive
Magna Business Park
Citywest Road
Dublín 24
Irlanda
Responsable de la fabricación
Mundipharma DC B.V.
Leusderend 16
3832 RC Leusden
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien | Lietuva | |||
Mundipharma Comm. VA | Mundipharma Corporation (Ireland) Limited | |||
+32 2 358 54 68 | Airija | |||
Tel +353 1 206 3800 | ||||
Luxembourg/Luxemburg | ||||
??„?????????? ??????? ???“ | Mundipharma Comm. VA | |||
???.: + 359 2 962 13 56 | +32 2 358 54 68 | |||
e-mail: [email protected] | ||||
Ceská republika | Magyarország |
Mundipharma GesmbH Austria - organizacnísložka | Medis Hungary Kft |
Tel: + 420 296 188 338 | Tel: +36 23 801 028 |
E-Mail: [email protected] | |
Danmark | Malta |
Mundipharma A/S | Mundipharma Corporation (Ireland) Limited |
Tlf. +45 17 48 00 | L-Irlanda |
Tel +353 1 206 3800 |
Deutschland | Nederland | ||
Mundipharma GmbH | Mundipharma Pharmaceuticals B.V. | ||
Gebührenfreie Info-Line: +49 69 506029-000 | Tel: + 31 (0)33 450 82 70 | ||
Eesti | Norge |
Mundipharma Corporation (Ireland) Limited | Mundipharma AS |
L-Irlanda | Tlf: + 47 67 51 89 00 |
Tel +353 1 206 3800 |
Ελλ?δα | Österreich | |
Mundipharma Corporation (Ireland) Limited | Mundipharma Gesellschaft m.b.H. | |
Ιρλανδ?α | Tel: +43 (0)1 523 25 05 | |
Tel +353 1 206 3800 |
España | Polska | ||
Mundipharma Pharmaceuticals, S.L. | Mundipharma Polska Sp. z o.o. | ||
Tel: +34 91 3821870 | Tel: + (48 22) 3824850 | ||
France | Portugal | ||
MUNDIPHARMA SAS | Mundipharma Farmacêutica Lda | ||
+33 1 40 65 29 29 | Tel: +351 21 901 31 62 | ||
Hrvatska | România | ||
Medis Adria d.o.o. | Mundipharma Gesellschaft m.b.H., Austria | ||
Tel: + 385 (0) 1 230 34 46 | Tel: +40751 121 222 | ||
Ireland | Slovenija | |
Mundipharma Pharmaceuticals Limited | Medis, d.o.o. | |
Tel +353 1 206 3800 | Tel: +386 158969 00 | |
Ísland | Slovenská republika | |
Icepharma hf. | Mundipharma Ges.m.b.H.-o.z. | |
Tlf: + 354 540 8000 | Tel: + 4212 6381 1611 | |
Italia | Suomi/Finland | ||
Mundipharma Pharmaceuticals Srl | Mundipharma Oy | ||
Tel: +39 02 3182881 | Puh/Tel: + 358 (0)9 8520 2065 | ||
Κ?προς | Sverige | ||
Mundipharma Pharmaceuticals Ltd | Mundipharma AB | ||
Τηλ.: +357 22 815656 | Tel: + 46 (0)31 773 75 30 | ||
Latvija | ||
SIA Inovativo biomedicinas tehnologiju instituts | ||
Tel: + 37167800810 | ||
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NYXOID 1,8 MG SOLUCION PARA PULVERIZACION NASAL EN ENVASE UNIDOSISForma farmacéutica: INYECTABLE, 0,4 mg/mlPrincipio activo: NaloxonaFabricante: Medochemie LimitedRequiere recetaForma farmacéutica: INYECTABLE, 0,4 mg/mlPrincipio activo: NaloxonaFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 0,4 mg/mlPrincipio activo: NaloxonaFabricante: B. Braun Melsungen AgRequiere receta
Médicos online para NYXOID 1,8 MG SOLUCION PARA PULVERIZACION NASAL EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NYXOID 1,8 MG SOLUCION PARA PULVERIZACION NASAL EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes