

НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій

Запитайте лікаря про рецепт на НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій

Інструкція із застосування НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій
Введення
Опис: інформація для користувача
NUCEIVA 50 Одиниць порошок для ін'єкційного розчину
ботулінова токсина типу А
Всім пацієнтам рекомендується уважно прочитати цю брошуру перед початком використання цього лікарського засобу, оскільки вона містить важливу інформацію для вас.
- Збережіть цю брошуру, оскільки вам може знадобитися знову її прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лікарський засіб призначений тільки для вас, і його не слід давати іншим людям, навіть якщо вони мають такі самі симптоми, як і ви, оскільки це може їм нашкодити.
- Якщо ви відчуваєте будь-які побічні ефекти, зверніться до вашого лікаря або фармацевта, навіть якщо це можливі побічні ефекти, які не вказані в цій брошурі. Див. розділ 4.
Зміст брошури
- Що таке NUCEIVA і для чого вона використовується
- Що вам потрібно знати перед початком використання NUCEIVA
- Як використовувати NUCEIVA
- Можливі побічні ефекти
- Зберігання NUCEIVA
- Зміст упаковки та додаткова інформація
1. Що таке NUCEIVA і для чого вона використовується
NUCEIVA містить активний інгредієнт ботулінову токсину типу А.
Вона запобігає скороченню м'язів, що призводить до тимчасової паралічу. Вона діє шляхом блокування нервових імпульсів до м'язів, у які вона була введена.
NUCEIVA використовується для тимчасового покращення вигляду вертикальних зморшок між бровами. Вона використовується у дорослих молодших 65 років, у яких зморшки на обличчі мають значний психологічний вплив.
2. Що вам потрібно знати перед початком використання NUCEIVA
Не використовуйте NUCEIVA:
- якщо ви алергічні на ботулінову токсину типу А або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6);
- якщо ви страждаєте на міастенію або синдром Еатона-Ламберта (хронічні захворювання, які впливають на м'язи);
- якщо у вас є інфекція або запалення в місцях ін'єкції між бровами і над ними (як показано на малюнку 1).
Попередження та застереження
Дуже рідко можуть виникати побічні ефекти, які можуть бути пов'язані з поширенням ботулінової токсини від місця ін'єкції (наприклад, м'язова слабкість, труднощі з ковтанням або потраплянням їжі чи рідини в дихальні шляхи). Пацієнти, які отримують рекомендуємі дози, можуть відчувати надмірну м'язову слабкість.
Ін'єкція була пов'язана з місцевим болем, запаленням/набуханням, аномальною чутливістю (парестезією), зниженням чутливості (гіпостезією), болем при пальпації, висипом на шкірі (еритемою), місцевою інфекцією, кровотечею та/або гематомами. Біль і/або тривога, пов'язані з голкою, призвели до вазовагальних реакцій, таких як блідість, нудота, потіння, розмитість зору, швидка серцебиття, головокружіння та/або тимчасове зниження артеріального тиску, яке призводить до головокружіння або непритомності.
Відвідайте вашого лікаря негайно, якщо у вас виникли труднощі з ковтанням, мовленням або диханням після лікування.
- Цей лікарський засіб не рекомендується пацієнтам, які мали проблеми з ковтанням (дисфагією) і диханням недавно або в минулому, оскільки це може перешкодити безпечному введенню лікарського засобу на думку лікаря.
- Занадто часте або надмірне введення може призвести до утворення антитіл. Утворення антитіл може перешкодити дії ботулінової токсини типу А навіть для інших застосувань.
- Дуже рідко може виникнути алергічна реакція після ін'єкції ботулінової токсини.
Серед симптомів включають шкірні реакції, такі як кропив'янка, свербіж і шкіра, червона або бліда, набухання очей, губ, рота або горла, слабкий і швидкий пульс, головокружіння і свистіння або задуха
- Може виникнути опущення повіки після лікування.
Повідомте вашого лікаря, якщо:
- у вас були проблеми з попередніми ін'єкціями ботулінової токсини;
- ви не спостерігали жодних значних покращень зморшок через місяць після першого циклу лікування;
- ви страждаєте на певні захворювання, які впливають на нервову систему (наприклад, боковий аміотрофічний склероз або моторна нейропатія);
- у вас є запалення в місці ін'єкції;
- м'язи, які будуть ін'єктовані, слабкі або пошкоджені;
- у вас є кровотечний розлад, оскільки ін'єкція може призвести до гематом.
Діти та підлітки
Не рекомендується використовувати цей лікарський засіб у дітей молодших 18 років.
Інші лікарські засоби та NUCEIVA
Повідомте вашого лікаря або фармацевта, якщо ви приймаєте, недавно приймали або можете приймати інші лікарські засоби.
Не рекомендується використовувати ботулінову токсину в поєднанні з антибіотиками аміноглюкозидами, спектиноміцином або іншими лікарськими засобами, які впливають на нервові імпульси до м'язів.
Повідомте вашого лікаря, якщо вам недавно було введено лікарський засіб, який містить ботулінову токсину (активний інгредієнт NUCEIVA), оскільки це може надмірно збільшити дію цього лікарського засобу.
Вагітність та лактація
Якщо ви вагітні або годуєте грудьми, вважаєте, що можете бути вагітною або плануєте завагітніти, проконсультуйтеся з вашим лікарем перед використанням цього лікарського засобу.
Не рекомендується використовувати цей лікарський засіб під час вагітності чи у жінок, які можуть завагітніти і не використовують контрацепцію.
Цей лікарський засіб не рекомендується під час лактації.
Водіння транспортних засобів та використання машин
Відзначені м'язова слабкість, головокружіння та порушення зору при використанні цього лікарського засобу, які можуть зробити водіння чи використання машин небезпечним. Не водьте транспортні засоби та не використовуйте машини, доки ці ефекти не зникнуть.
NUCEIVA містить натрій
Цей лікарський засіб містить менше 1 ммоль натрію (23 мг) на дозу; тобто, він практично не містить натрію.
3. Як використовувати NUCEIVA
Одніичні дози NUCEIVA не можна заміняти з дозами, використовуваними для інших препаратів ботулінової токсини.
Цей лікарський засіб повинен бути введений тільки лікарями з відповідною кваліфікацією та досвідом лікування зморшок між бровами.
Звичайна доза NUCEIVA становить 20 одиниць. Вам буде введено рекомендований об'єм 0,1 мл (4 одиниці) цього лікарського засобу в кожне з 5 місць ін'єкції.
Покращення глибини зморшок між бровами зазвичай відбувається через кілька днів після лікування.
Інтервал між лікуваннями визначатиме ваш лікар.
Як вводиться NUCEIVA
Цей лікарський засіб вводиться в м'язи (внутрішньом'язово), безпосередньо в уражену ділянку між бровами та над ними.
Після відновлення NUCEIVA слід використовувати тільки для лікування одного пацієнта під час однієї сесії.
Якщо у вас є будь-які питання щодо використання цього продукту, проконсультуйтеся з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може призвести до побічних ефектів, хоча не всі люди їх відчувають.
Загалом, побічні ефекти виникають у перші дні після ін'єкції та є тимчасовими. Більшість побічних ефектів мають легку або середню інтенсивність.
Якщо у вас виникли труднощі з диханням, ковтанням або мовленням після отримання цього лікарського засобу, зверніться до вашого лікаря негайно.
Якщо ви спостерігали набухання, свербіж, набухання обличчя або горла, свистіння, відчуття слабкості та задуху, зверніться до вашого лікаря негайно, оскільки ці симптоми можуть бути ознаками алергічної реакції.
Ймовірність виникнення побічного ефекту описується в наступних категоріях:
Часті (можуть виникнути у до 1 з 10 осіб) | Головний біль, м'язова слабість, опущення повіки, гематоми в місці ін'єкції |
Нечасті (можуть виникнути у до 1 з 100 осіб) | Розлади чутливості, головний біль, сухість очей, набухання повік, набухання очей, м'язові скорочення, місце ін'єкції: червоність, біль, оніміння |
Повідомлення про побічні ефекти
Якщо ви відчуваєте побічні ефекти, зверніться до вашого лікаря або фармацевта, навіть якщо це можливі побічні ефекти, які не вказані в цій брошурі. Ви також можете повідомити про них безпосередньо через національну систему повідомлень, включену до додатку V. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання NUCEIVA
Зберігати в холодильнику (між 2°C та 8°C)
Тримати поза зоною видимості та досягнення дітей.
Флакон без відкриття
Не використовувати цей лікарський засіб після закінчення терміну придатності, вказаного на флаконі та коробці після CAD.
6. Зміст упаковки та додаткова інформація
Склад NUCEIVA
- Активний інгредієнт: 50 одиниць ботулінової токсини типу А.
- Інші компоненти: людська альбумін та хлорид натрію.
Вигляд продукту та вміст упаковки
NUCEIVA випускається у вигляді білого порошку для ін'єкційного розчину в скляному флаконі.
Кожна упаковка містить 1 флакон.
Власник реєстраційного посвідчення та виробники
Evolus Pharma B.V.
Apollolaan 151
1077 AR Amsterdam
Нідерланди
Ви можете запитати додаткову інформацію про цей лікарський засіб, звернувшись до місцевого представника власника реєстраційного посвідчення.
Дата останнього перегляду цієї брошури: травень 2024 року.
ЦЯ ІНФОРМАЦІЯ ПРИДНАНА ТІЛЬКИ ЛІКАРЯМ ТА ПРАЦІВНИКАМ МЕДИЧНОЇ СПРАВИ:
Одніичні дози ботулінової токсини не можна заміняти з дозами інших препаратів ботулінової токсини. Рекомендовані дози в одиницях відрізняються від доз інших препаратів ботулінової токсини.
Відновлення слід проводити згідно з хорошими клінічними практиками, особливо щодо асептичної техніки. NUCEIVA відновлюється з хлоридом натрію 9 мг/мл (0,9%) для ін'єкційного розчину. Витягують 1,25 мл хлориду натрію 9 мг/мл (0,9%) для ін'єкційного розчину, щоб отримати відновлений ін'єкційний розчин у концентрації 4 одиниці/0,1 мл.
Кількість розчинника, доданого до флакону 50 одиниць (хлорид натрію 9 мг/мл (0,9%) для ін'єкційного розчину) | Результируюча доза (одиниці на 0,1 мл) |
1,25 мл | 4,0 одиниці |
Центральна частина гумової пробки повинна бути очищена спиртом. Введіть повільно розчинник у флакон через пробку та оберніть флакон, уникайте утворення бульбашок. Флакон слід викинути, якщо при створенні вакууму розчинник не вводиться у флакон. Після відновлення ін'єкційний розчин слід візуально перевірити перед використанням, щоб переконатися, що це прозорий та безбарвний розчин без частинок.
Відновлений NUCEIVA (50 одиниць/1,25 мл) вводиться за допомогою стерильної голки калібру 30. Вводять 4 одиниці (4 одиниці/0,1 мл) у кожне з 5 місць ін'єкції (див. малюнок 1): 2 ін'єкції у кожний м'яз-корректор (внутрішня нижня та внутрішня верхня поверхня) та 1 ін'єкція у м'яз-проріз, що становить загальну дозу 20 одиниць.
Малюнок 1 Місця ін'єкції
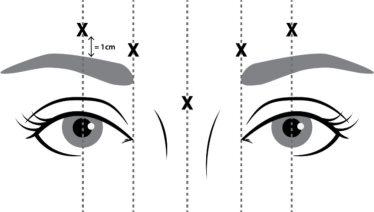
Для зменшення ускладнення опущення повіки слід вжити наступні заходи:
- Уникайте ін'єкції поблизу підіймача верхньої повіки, особливо у пацієнтів з великими депресорами брови.
- Ін'єкції у корректорну частину повинні застосовуватися не менше 1 см над орбітальним краєм.
- Перевірте, щоб об'єм/доза, введена була точною, та якщо можливо, мінімальною.
Процедура безпечної утилізації флаконів, шприців та матеріалів, що використовувалися:
Негайно після використання відновлений ін'єкційний розчин NUCEIVA, який залишився у флаконі або шприці, слід інактивувати перед утилізацією 2 мл розчину гіпохлориту натрію, розбавленого до 0,5% або 1% хлору. Після інактивації викинути згідно з місцевими вимогами.
Флакони, шприці та матеріали, що використовувалися, не слід викидати та слід утилізувати у відповідних контейнерах та викинути згідно з місцевими правилами.
Рекомендації у разі аварії під час обробки ботулінової токсини:
У разі аварії під час обробки продукту, як у сухому, так і у відновленому вигляді, слід негайно застосувати відповідні заходи, описані нижче.
- Токсин дуже чутливий до тепла та певних хімічних речовин.
- Розливи слід очищати матеріалом, насиченим розчином гіпохлориту натрію (білого вапна), у разі сухого продукту або матеріалом, насиченим сухим матеріалом, якщо продукт вже відновлений.
- Забруднені поверхні слід очищати матеріалом, насиченим розчином гіпохлориту натрію (білого вапна), та висушувати.
- Якщо флакон розбився, слід обережно зібрати уламки скла та очистити продукт, як вказано вище, уникайте порізів шкіри.
- У разі бризг слід промити розчином гіпохлориту натрію та ретельно промити водою.
- У разі бризг у очі слід ретельно промити водою або офтальмологічним розчином.
- Якщо оператор поранився (поріз himself, уколов), слід виконати вище вказані дії та вжити відповідні медичні заходи, залежно від введеної дози.
Слід суворо дотримуватися цих інструкцій щодо використання, обробки та утилізації.
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкційФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 200 ОД/млДіючі речовини: botulinum toxinВиробник: Ipsen PharmaПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 125 Одиниць СпейвудДіючі речовини: botulinum toxinВиробник: Ipsen Pharma S.A.U.Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 100 одиницьДіючі речовини: botulinum toxinВиробник: Merz Pharmaceuticals GmbhПотрібен рецепт
Аналоги НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій у Польща
Аналог НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій у Україна
Лікарі онлайн щодо НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на НУЦЕЇВА 50 одиниць порошок для приготування розчину для ін'єкцій – за рішенням лікаря та згідно з місцевими правилами.














