
MOVYMIA 20 MICROGRAMOS/80 MICROLITROS SOLUCIÓN INYECTABLE

Cómo usar MOVYMIA 20 MICROGRAMOS/80 MICROLITROS SOLUCIÓN INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Movymia 20 microgramos/80 microlitros solución inyectable
teriparatida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Movymia y para qué se utiliza
- Qué necesita saber antes de empezar a usar Movymia
- Cómo usar Movymia
- Posibles efectos adversos
- Conservación de Movymia
- Contenido del envase e información adicional
1. Qué es Movymia y para qué se utiliza
Movymia contiene el principio activo teriparatida, que es empleado para aumentar la fortaleza del hueso y reducir el riesgo de fracturas mediante la estimulación de la formación de hueso.
Movymia se usa para el tratamiento de la osteoporosis en adultos. La osteoporosis es una enfermedad que hace que sus huesos se desgasten y se vuelvan frágiles. Esta enfermedad es especialmente frecuente en las mujeres después de la menopausia, pero también puede ocurrir en varones. La osteoporosis también es frecuente en pacientes tratados con medicamentos denominados corticosteroides.
2. Qué necesita saber antes de empezar a usar Movymia
No use Movymia
- si es alérgico a teriparatida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene niveles de calcio elevados en la sangre (hipercalcemia preexistente).
- si padece problemas graves de riñón.
- si alguna vez ha tenido cáncer de huesos o si otros tipos de cáncer se han extendido (metastatizado) a sus huesos.
- si tiene determinadas enfermedades de los huesos. Si tiene una enfermedad de los huesos consulte a su médico.
- si tiene niveles elevados de fosfatasa alcalina en sangre sin explicación aparente, lo cual podría indicar que padece la enfermedad de Paget en el hueso (enfermedad con cambios anormales del hueso). Si no está seguro, consulte a su médico.
- si ha recibido radioterapia que haya podido afectar a sus huesos.
- si está embarazada o en la lactancia.
Advertencias y precauciones
Movymia puede aumentar el calcio en su sangre u orina.
Consulte a su médico antes o mientras esté utilizando Movymia:
- Si usted tiene continuamente náuseas, vómitos, estreñimiento, baja energía o debilidad muscular dígaselo a su médico. Estos pueden ser síntomas de que hay demasiado calcio en su sangre.
- Si usted sufre de piedras en el riñón o ha tenido piedras en el riñón.
- Si usted sufre de problemas de riñón (insuficiencia renal moderada) debe decírselo a su médico.
Algunos pacientes, tras las primeras dosis de Movymia, sufren mareos o aumento de la frecuencia cardiaca. Para las primeras dosis, utilice Movymia en un lugar donde pueda sentarse o tumbarse inmediatamente si se marea.
El tiempo de tratamiento recomendado de 24 meses no debe ser excedido.
Antes de insertar un cartucho en Movymia Pen anote el número de lote (Lote) del cartucho y la fecha de la primera inyección en la caja del cartucho y proporcione esta información cuando comunique cualquier reacción adversa.
Movymia no debe utilizarse en adultos en crecimiento.
Niños y adolescentes
Movymia no debe utilizarse en niños y adolescentes (menores de 18 años).
Otros medicamentos y Movymia
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Esto es importante porque algunos medicamentos (p. ej. digoxina/digitálicos, un medicamento empleado para tratar enfermedades cardiacas) pueden interactuar con teriparatida.
Embarazo y lactancia
No utilice Movymia si está embarazada o en periodo de lactancia. Si usted es una mujer en edad fértil, debe utilizar métodos anticonceptivos eficaces durante el tratamiento con Movymia. Si se queda embarazada mientras está utilizando Movymia, debe interrumpirse el tratamiento. Consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Algunos pacientes pueden sentir mareos después de la inyección de Movymia. Si usted siente mareo no debe conducir o usar máquinas hasta que se encuentre mejor.
Movymia contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Movymia
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es de 20 microgramos (correspondientes a 80 microlitros) administrados una vez al día mediante una inyección debajo de la piel (inyección subcutánea) en el muslo o en el abdomen.
Para ayudarle a recordar inyectarse su medicamento, inyéctese sobre la misma hora cada día.
Movymia puede inyectarse a la hora de las comidas. Inyéctese Movymia cada día durante tanto tiempo como su médico se lo prescriba. La duración total del tratamiento con Movymia no debe exceder 24 meses. Usted no debe recibir más de un ciclo de 24 meses de tratamiento a lo largo de su vida.
Su médico puede aconsejarle que use Movymia con calcio y vitamina D. Su médico le indicará qué cantidad debe tomar cada día.
Movymia puede administrarse con o sin comida.
Los cartuchos de Movymia están diseñados para uso exclusivo con el sistema de administración multidosis reutilizable Movymia Pen y agujas de pluma compatibles. La pluma y agujas de inyección no están incluidas con Movymia. Sin embargo, para el inicio del tratamiento se debe utilizar un envase con cartucho y pluma, que contiene un estuche del cartucho de Movymia y un estuche de Movymia Pen.
La pluma puede utilizarse con agujas de inyección desarrolladas según la norma ISO de agujas de pluma de un calibre entre 29 G y 31 G (diámetro de 0,25 – 0,33 mm) y una longitud entre 5 mm y 12,7 mm sólo para la inyección subcutánea.
Antes de la primera utilización, inserte el cartucho en la pluma. Para la correcta utilización de este medicamento es muy importante que siga atentamente las instrucciones de uso detalladas de la pluma, provistas con esta.
Use una nueva aguja de inyección para cada inyección a fin de prevenir la contaminación y deseche de forma segura la aguja después de su utilización.
Nunca guarde la pluma con la aguja colocada.
Nunca comparta su pluma con otras personas.
No use Movymia Pen para inyectar cualquier otro medicamento (p. ej., insulina). La pluma está diseñada para su uso con Movymia exclusivamente.
No rellene el cartucho.
No transfiera el medicamento a una jeringa.
Debe inyectar Movymia al poco tiempo de haber sacado de la nevera la pluma con el cartucho insertado. Ponga de nuevo en la nevera la pluma con el cartucho insertado inmediatamente después de haberla utilizado. No retire el cartucho de la pluma después de cada uso. Consérvelo en el porta cartuchos durante el periodo de tratamiento completo de 28 días.
Preparación de la pluma para su uso
- Para garantizar la correcta administración de Movymia lea siempre las Instrucciones de uso de Movymia Pen, incluidas en el estuche de la pluma.
- Lávese las manos antes de manipular el cartucho o la pluma.
- Compruebe la fecha de caducidad en la etiqueta del cartucho antes de insertarlo en la pluma.
Asegúrese de que falten al menos 28 días hasta la fecha de caducidad. Inserte el cartucho en la pluma antes de la primera utilización como se detalla en las instrucciones de la pluma. Anote el número de lote (Lote) de cada cartucho y su primera fecha de inyección en un calendario.
También debe apuntarse la fecha de la primera inyección en el estuche de Movymia (ver el espacio provisto en la caja: {Primera utilización:}).
- Después de insertar un nuevo cartucho y antes de la primera inyección de dicho cartucho prepare la pluma según las instrucciones provistas. No vuelva a prepararla tras la primera dosis.
Inyección de Movymia
- Antes de inyectar Movymia, limpie la piel donde piensa inyectarla (muslo o abdomen) como se lo haya indicado el médico.
- Pellizque con suavidad la piel que ha limpiado e inserte la aguja de forma perpendicular a la piel. Presione el botón y manténgalo presionado hasta que el indicador de dosis haya vuelto a la posición de inicio.
- Tras la inyección, deje la aguja en la piel durante seis segundos para asegurarse de recibir la dosis completa.
- En cuanto haya finalizado la inyección, coloque el capuchón de protección de la aguja en la aguja de la pluma; enrosque el capuchón en sentido contrario al de las agujas del reloj para retirar la aguja de la pluma. Esto mantendrá la esterilidad del Movymia restante y evitará fugas de la pluma. También evitará que se introduzca de nuevo aire en el cartucho y que la aguja se obstruya.
- Vuelva a colocar el capuchón en la pluma. Deje el cartucho en la pluma.
Si usa más Movymia del que debe
Si por error se ha administrado más cantidad de Movymia de la prescrita, consulte a su médico o farmacéutico.
Los efectos que pueden esperarse de una sobredosis incluyen náuseas, vómitos, mareos y dolor de cabeza.
Si olvidó usar Movymia
Si olvida una inyección o no puede inyectarse su medicamento a la hora habitual, hágalo tan pronto como pueda ese mismo día. No use una dosis doble para compensar las dosis olvidadas. No se inyecte más de una vez en el mismo día.
Si interrumpe el tratamiento con Movymia
Si está pensando interrumpir el tratamiento con Movymia, por favor consulte con su médico. Su médico le aconsejará y decidirá sobre cuánto tiempo debe ser tratado con Movymia.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos más frecuentes son dolor en las extremidades (muy frecuentes, pueden afectar a más de 1 de cada 10 pacientes). Otros efectos adversos frecuentes (que afectan a hasta 1 de cada 10 pacientes) son malestar, dolor de cabeza y mareo. Si se marea después de una inyección, siéntese o túmbese hasta que se encuentre mejor. En caso de no mejorar, consulte a su médico antes de continuar con el tratamiento. Ha habido casos de desmayo tras el uso de teriparatida.
Si tiene molestias alrededor de la zona de inyección como enrojecimiento de la piel, dolor, hinchazón, picor, hematomas o ligero sangrado (que pueden ocurrir frecuentemente), éstas deberían desaparecer en unos días o semanas. Si no es así, dígaselo a su médico.
Rara (pueden afectar hasta 1 de cada 1 000 pacientes), algunos pacientes pueden experimentar reacciones alérgicas, que consisten en dificultad para respirar, hinchazón de la cara, erupción cutánea y dolor en el pecho. Normalmente estas reacciones tienen lugar justo después de la inyección. En raras ocasiones, pueden producirse reacciones alérgicas graves y potencialmente mortales, incluyendo anafilaxia.
Otros efectos adversos son:
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes):
- aumento de los niveles de colesterol en sangre
- depresión
- dolor neuropático en la pierna
- sensación de desvanecimiento
- sensación de que todo da vueltas
- palpitaciones irregulares
- dificultad para respirar
- aumento de la sudoración
- calambres musculares
- pérdida de energía
- cansancio
- dolor de pecho
- tensión arterial baja
- acidez de estómago (dolor o sensación de ardor justo debajo del esternón)
- vómitos
- hernia del tubo que lleva la comida hasta su estómago (hernia de hiato)
- hemoglobina baja o bajo recuento de glóbulos rojos (anemia)
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes):
- aumento de la frecuencia cardiaca
- sonido anormal del corazón
- falta de aliento
- almorranas (hemorroides)
- pérdida de orina
- aumento de la necesidad de orinar
- aumento de peso
- piedras en el riñón
- dolor en los músculos y en las articulaciones. Algunos pacientes han tenido calambres en la espalda graves o dolor y tuvieron que ingresar en el hospital.
- aumento en los niveles de calcio en sangre
- aumento de los niveles de ácido úrico en sangre
- aumento en los niveles de una enzima llamada fosfatasa alcalina
Raros(pueden afectar hasta 1 de cada 1.000 pacientes):
- reducción de la función del riñón, incluyendo insuficiencia renal
- hinchazón, principalmente en las manos, pies y piernas
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Movymia
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y el cartucho después de CAD y EXP respectivamente. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). No congelar. Mantener el cartucho en el estuche para protegerlo de la luz.
Puede utilizar Movymia durante 28 días después de realizar la primera inyección mientras el cartucho / la pluma con el cartucho insertado se conserve en nevera (entre 2 ºC y 8 ºC).
Evite colocar el cartucho cerca del congelador de la nevera para prevenir su congelación. No use Movymia si está o ha estado congelado.
Cada cartucho debe desecharse de forma adecuada después de 28 días del primer uso, aunque no esté vacío del todo.
Movymia contiene una solución transparente e incolora. No utilice Movymia si tiene partículas sólidas o si la solución está turbia o presenta color.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Movymia
- El principio activo es teriparatida. Cada dosis de 80 microlitros contiene 20 microgramos de teriparatida. Un cartucho de 2,4 ml contiene 600 microgramos de teriparatida (correspondientes a 250 microgramos por ml).
- Los demás componentes son: ácido acético glacial, manitol, metacresol, acetato de sodio trihidrato, ácido clorhídrico (para ajustar el pH), hidróxido de sodio (para ajustar el pH) y agua para preparaciones inyectables (ver sección 2 “Movymia contiene sodio”).
Aspecto del producto y contenido del envase
Movymia es una solución inyectable (inyectable) transparente e incolora. Se presenta en un cartucho. Cada cartucho contiene 2,4 ml de solución suficiente para 28 dosis.
Tamaños de envase: 1 cartucho o 3 cartuchos envasados en una bandeja de plástico sellada con tapa y envasada en un estuche.
Envase con cartucho y pluma de Movymia: 1 cartucho de Movymia envasado en una bandeja de plástico sellada con tapa y envasada en un estuche y 1 Movymia Pen envasada en un estuche.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Alemania
Responsable de la fabricación
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungría
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Laboratorio STADA, S.L.
Tel: +34 934738889
Fecha de la última revisión de este prospecto:Septiembre 2021.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/
La información detallada de este producto está también disponible escaneando el código QR que figura a continuación o el estuche con un smartphone. La misma información también está disponible en la siguiente URL: movymiapatients.com
Instrucciones de uso
Movymia Pen
Pluma para inyección reutilizable para su uso con los cartuchos de Movymia, para inyección subcutánea
Cuando utilice la Movymia Pen, siga siempre las instrucciones que se dan a continuación y en el reverso.
Partes de la Movymia Pen
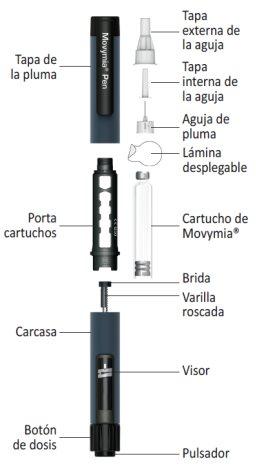
Preparación de la pluma - Primera utilización /cambio de cartuchos
Siga las instrucciones cada vez que coloque un cartucho de Movymia nuevo en la Movymia Pen. No repita este paso antes de cada inyección diaria, ya que de lo contrario no tendrá suficiente Movymia para 28 días.
Lea el prospecto del cartucho de Movymia que se da por separado.
A: Retire la tapa de la pluma.
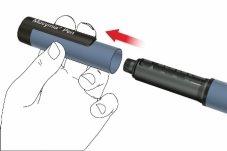
B: Gire el porta cartuchos para retirarlo (acoplamiento de bayoneta).

C: Si desea cambiar el cartucho, saque el cartucho vacío. Coloque un cartucho de Movymia nuevo en el porta cartuchos con la tapa metálica plegable del cartucho en primer lugar.
Anote la fecha de la primera inyección de cada nuevo cartucho. Esto le ayudará a saber cuándo se acaban las 28 dosis diarias por cartucho.
D: Empuje con el dedo la varilla roscada, cuidadosamente, en línea recta y hasta el tope. Esto no es necesario cuando la varilla ya se encuentra en posición inicial, como la primera vez que se utiliza. La varilla roscada no puede introducirse por completo en la carcasa de la pluma.
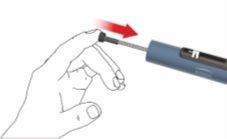
E: Acople el porta cartuchos a la carcasa girándolo 90 grados, hasta el tope.

F: Acople una nueva aguja de la pluma como sigue:
- Retire la lámina desplegable.
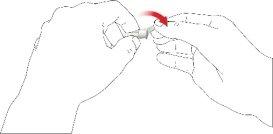
- Enrosque la aguja de la pluma en sentido horario en el porta cartuchos. Asegúrese de que la aguja de la pluma esté correctamente colocada y se apoye firmemente en el porta cartuchos.
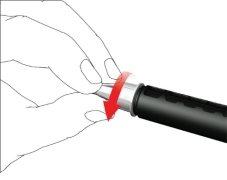
- Retire la tapa externa de la aguja y guárdela.
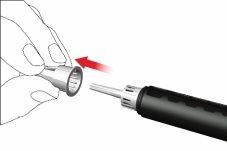
- Retire y deseche la tapa interna de la aguja.
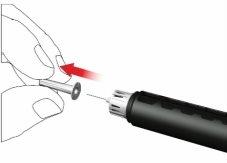
Al acoplar la aguja, es posible que se escapen algunas gotas; esto es normal.
G: Cebado
La pluma debe cebarse y probarse antes de introducir un nuevo cartucho y antes de la primera inyección de cada cartucho.
- Gire el botón de dosis en sentido horario hasta que vea el símbolo de una gota en el visor de dosis. Asegúrese de que las dos líneas del indicador estén alineadas. Durante el ajuste de dosis, la pluma emite un clic y ofrece una resistencia perceptible.
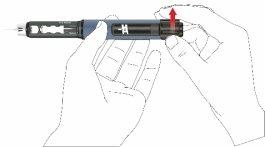
- Sostenga la pluma con la aguja apuntando hacia arriba.
- Presione el pulsador hasta el fondo. Manténgalo presionado hasta que la indicación de dosis regrese a la posición inicial. Algunas gotas del medicamento deben salir de la punta de la aguja.
De lo contrario, repita el paso G hasta que vea algunas gotas. No repita el paso G más de cuatro veces; en caso necesario, siga las instrucciones de la sección Solución de problemas en el reverso.
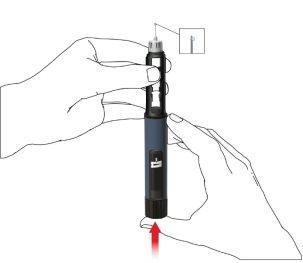
Administración mediante la Movymia Pen
Lávese las manos con jabón cuidadosamente para reducir el riesgo de infección.
Asegúrese de tener a mano:
- la Movymia Pen con el cartucho insertado
- una aguja compatible con la pluma
- un recipiente para objetos punzantes resistente a perforaciones para las agujas usadas.
No utilicela pluma si el cartucho está turbio o coloreado o contiene partículas.
Lea el prospecto del cartucho de Movymia que se da por separado.
- Coloque la aguja de la pluma
Utilice una aguja nueva para cada inyección. No utilice la aguja de la pluma si el embalaje está dañado o usted no lo abrió.
Nota:no es necesario cambiar la aguja cuando se usa inmediatamente después de la preparación de la pluma. En este caso, continúe con el paso “2. Ajuste de la dosis e inyección”.
- Retire la lámina desplegable.
- Enrosque la aguja de la pluma en sentido horario en el porta cartuchos. Asegúrese de que la aguja de la pluma esté correctamente colocada y se apoye firmemente en el porta cartuchos.
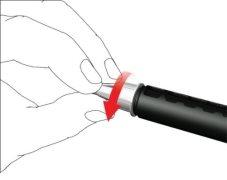
- Retire la tapa externa de la aguja y guárdela.
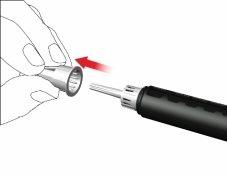
- Retire y deseche la tapa interna de la aguja.
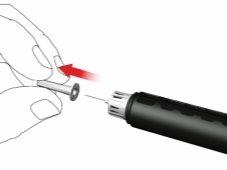
Al acoplar la aguja, es posible que se escapen algunas gotas; esto es normal.
- Ajuste de la dosis e inyección
Advertencia: asegúrese de utilizar el líquido del fármaco correcto. Verifique la etiqueta del cartucho antes de insertarlo en el porta cartuchos.
- Para ajustar la dosis diaria fija de 80 microlitros, gire el botón de dosis en sentido horario hasta que se detenga y no pueda seguir girando. Asegúrese de que en el visor aparezca un símbolo de flecha y de que esté alineada con la línea indicadora. Durante el ajuste de dosis, la pluma emite un clic y ofrece una resistencia perceptible. No intente forzar el botón de dosis.
Nota:si el cartucho contiene menos de 80 microlitros, el botón de dosis no podrá girarse en sentido horario hasta el símbolo de flecha. En ese caso, retire la tapa de la pluma, cambie el cartucho y realice un cebado siguiendo los pasos de preparación de la pluma.
- Seleccione un lugar de inyección adecuado y prepare la piel según las recomendaciones del médico. Tome con cuidado un pliegue de piel entre los dedos índice y pulgar. Inserte la aguja de forma recta y con cuidado en la piel, tal como se muestra en la ilustración.
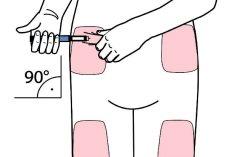
Advertencia:evite doblar o romper la aguja de la pluma. No incline la pluma después de insertar la aguja en la piel. Inclinar la pluma puede doblar o romper la aguja. Las agujas rotas pueden quedarse metidas en la piel. Si una aguja rota queda metida en la piel, consulte al médico de inmediato.
- Presione el pulsador hasta que la indicación de dosis regrese a la posición inicial. Deje la aguja en el pliegue de piel durante 6 segundos más.
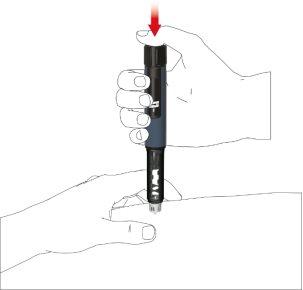
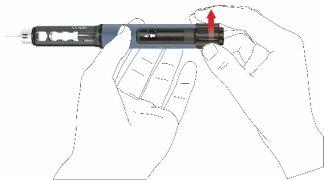
- Saque la pluma lentamente. Compruebe si el visor se encuentra en la posición inicial para asegurarse de que se haya inyectado la dosis completa.

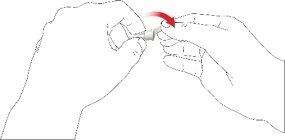
- Retirar la aguja de la pluma
- Con cuidado, coloque la tapa externa de la aguja sobre la aguja de la pluma.
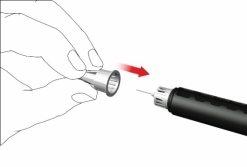
- Enrosque la tapa de la aguja en sentido antihorario para retirar la aguja de la pluma. Deséchela correctamente, por ejemplo en un recipiente para objetos punzantes resistente a perforaciones.
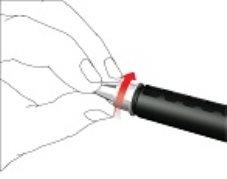
- Vuelva a colocar la tapa de la pluma
- No retire el cartucho de la Movymia Pen antes de vaciarla.
- Vuelva a colocar la tapa de la pluma después de cada uso.
- Meta la Movymia Pen con el cartucho en la nevera a una temperatura de 2 a 8 °C inmediatamente después de utilizarla.
Nota para profesionales sanitarios
Las políticas locales, de los profesionales sanitarios o de la institución pueden reemplazar las instrucciones con respecto a la manipulación y la eliminación de agujas.
Información adicional
La pluma de dosis fija reutilizable está diseñada para una fácil administración de Movymia para tratar la osteoporosis. Cada cartucho de Movymia contiene 28 dosis fijas de 80 microlitros de Movymia.
Utilice la Movymia Pen únicamente como le haya indicado el médico y según la información de estas instrucciones de uso y del prospecto de Movymia.
La Movymia Pen puede ser utilizada por pacientes que se inyectan y tienen más de 18 años de edad, profesionales sanitarios o terceros como, por ejemplo, familiares adultos.
La Movymia Pen no debe ser utilizada por pacientes ciegos o con discapacidad visual sin la ayuda de una persona físicamente capaz debidamente capacitada. Consulte a su médico en caso de tener problemas de audición o manipulación.
Si tiene alguna pregunta con respecto al uso de la Movymia Pen, póngase en contacto con nuestro servicio de atención al clienteen cualquier momento.
Número de teléfono: XXXXXXXXXXX
Correo electrónico: XXXXXXXXXXX
Agujascompatibles con la pluma
- Ypsomed mylife™ Clickfine®, calibre 29 a 31 (diámetro 0,25 – 0,33 mm) y longitud de 12, 10, 8 o 6 mm
- Agujas BD Micro-Fine Ultra™, calibre 29 a 31 (diámetro 0,25 – 0,33 mm) y longitud de 12,7, 8 o 5 mm
Las agujas para pluma de otros fabricantes pueden utilizarse de acuerdo con los detalles de compatibilidad indicados.
Las agujas de la pluma deben utilizarse solo una vezy solo una persona debe utilizar el cartucho de Movymia.
Almacenamiento y cuidado de la Movymia Pen
- Manipule la pluma con cuidado. No deje caer la pluma y evite golpearla contra superficies duras. Protéjala del agua, el polvo y la humedad.
- Puede utilizar un paño húmedo para limpiar la Movymia Pen. No utilice alcohol, disolventes o agentes de limpieza. No sumerja la Movymia Pen en agua, ya que podría dañarla.
- No utilice la Movymia Pen si está dañada o si tiene dudas sobre su correcto funcionamiento.
- Transporte y guarde la Movymia Pen con el cartucho colocado a la temperatura que se indica en el prospecto de Movymia que se da por separado.
- Guarde la Movymia Pen, los cartuchos y las agujas fuera del alcance de los niños.
- No guarde la Movymia Pen con la aguja acoplada, ya que podría causar la formación de burbujas de aire en el cartucho.
Cómo desechar la Movymia Pen y los accesorios utilizados
La Movymia Pen tiene una vida útil de dos años. Antes de desechar la Movymia Pen, retire la aguja de la pluma y el cartucho. Las agujas y los cartuchos utilizados deben desecharse de forma segura y por separado. La Movymia Pen puede desecharse de acuerdo con las instrucciones de las autoridades locales.
Advertencias
Siga las instrucciones presentadas en estas instrucciones de uso. De no seguirse estas instrucciones, existe riesgo de errores de medicación, dosis inadecuada, transmisión de enfermedades o infección. Si tiene algún problema de salud, solicite atención médica de inmediato.
Garantía
La garantía cubre los defectos materiales y de fabricación de la Movymia Pen durante dos años de uso a partir de la compra. Se limita al reemplazo de la pluma. La garantía no cubre daños causados por:
- el uso de cartuchos que no sean cartuchos de Movymia
- un uso, una manipulación o una limpieza incorrectos o negligentes
- un uso contrario al indicado en las instrucciones de uso
- la pluma utilizada con productos sanitarios, accesorios o consumibles distintos a aquellos mencionados en estas instrucciones de uso
- caídas, golpes, aplicación de fuerza, contacto con líquidos
- otros casos de exposición y desgaste que no sean conformes a las instrucciones de uso.
Solución de problemas
Si tiene alguna pregunta con respecto al uso de la Movymia Pen, siga las instrucciones que se dan en la tabla de la página siguiente:
Pregunta | Respuesta |
| Una pequeña burbuja de aire no afectará a la dosis ni causará daños. |
| Utilice otra aguja. Si la segunda aguja no puede colocarse, póngase en contacto con el servicio de atención al cliente. |
| Utilice otra aguja. |
| No utilice esa pluma; póngase en contacto con el servicio de atención al cliente. |
| Cambie la aguja y repita el cebado tal como se indica en las secciones de preparación de la pluma “F” y “G”. Si sigue sin salir medicamento, no utilice esa pluma; póngase en contacto con el servicio de atención al cliente. |
| La cantidad de Movymia restante en el cartucho es inferior a 80 microlitros. Cambie el cartucho y la aguja de la pluma y realice un cebado de conformidad con los pasos de preparación de la pluma. |
| No repita la inyección el mismo día. Utilice una aguja nueva para la inyección al día siguiente. Ajuste la dosis y efectúe la inyección como se describe en la sección “2. Ajuste de la dosis e inyección”. Si el visor sigue sin regresar a la posición inicial después de la inyección, no utilice esa pluma; póngase en contacto con el servicio de atención al cliente. |
| No utilice esa pluma; póngase en contacto con el servicio de atención al cliente. |
¿Cómo puedo restablecer el botón de dosis en la posición inicial? | No presione el pulsador. Para restablecer la pluma, simplemente gire el botón de dosis en sentido antihorario hasta la posición inicial. |
Distribuidor:
XXXXXXXXXX
Importador:
Gedeon Richter Plc.
Gyömroi út 19-21
1103 Budapest
Hungría
Representante Autorizado en la Comunidad Europea:
Ypsomed Distribution GmbH
Warmbacher Strasse 80
79618 Rheinfelden
Alemania
Fabricante legal:
Ypsomed AG
Brunnmattstrasse 6
3401 Burgdorf
Suiza
CE 0123
Fecha de revisión del texto:
- País de registro
- Precio medio en farmacia252.16 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MOVYMIA 20 MICROGRAMOS/80 MICROLITROS SOLUCIÓN INYECTABLEForma farmacéutica: INYECTABLE, 250 microgramos/mlPrincipio activo: teriparatideFabricante: Gp Pharm S.A.Requiere recetaForma farmacéutica: INYECTABLE, 250 µg/mlPrincipio activo: teriparatideFabricante: Eli Lilly Nederland B.V.Requiere recetaForma farmacéutica: INYECTABLE, 20 microgramos/80 microlitrosPrincipio activo: teriparatideFabricante: Theramex Ireland LimitedRequiere receta
Médicos online para MOVYMIA 20 MICROGRAMOS/80 MICROLITROS SOLUCIÓN INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MOVYMIA 20 MICROGRAMOS/80 MICROLITROS SOLUCIÓN INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











