LIVOGIVA 20 MICROGRAMOS/80 MICROLITROS SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar LIVOGIVA 20 MICROGRAMOS/80 MICROLITROS SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Livogiva 20 microgramos/80 microlitros solución inyectable en pluma precargada
teriparatida
Este medicamento esta´ sujeto a seguimiento adicional, lo que agilizara´ la deteccio´n de nueva informacio´n sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la seccio´n 4 incluye informacio´n sobre co´mo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Livogiva y para qué se utiliza
- Qué necesita saber antes de empezar a usar Livogiva
- Cómo usar Livogiva
- Posibles efectos adversos
- Conservación de Livogiva
- Contenido del envase e información adicional
1. Qué es Livogiva y para qué se utiliza
Livogiva contiene el principio activo teriparatida, que es empleado para aumentar la fortaleza del hueso y reducir el riesgo de fracturas mediante la estimulación de la formación de hueso.
Livogiva se usa para el tratamiento de la osteoporosis en adultos. La osteoporosis es una enfermedad que hace que sus huesos se desgasten y se vuelvan frágiles. Esta enfermedad es especialmente frecuente en las mujeres después de la menopausia, pero también puede ocurrir en varones. La osteoporosis también es frecuente en pacientes tratados con corticosteroides.
2. Qué necesita saber antes de empezar a usar Livogiva
No use Livogiva
- si es alérgico a teriparatida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene niveles de calcio elevados (hipercalcemia preexistente).
- si padece problemas graves de riñón.
- si alguna vez le han diagnosticado cáncer de huesos u otros tipos de cáncer que se hayan extendido (metastatizado) a sus huesos.
- si tiene determinadas enfermedades de los huesos. Si tiene una enfermedad de los huesos consulte a su médico.
- si tiene niveles elevados de fosfatasa alcalina en sangre sin explicación aparente, lo cual podría indicar que padece la enfermedad de Paget en el hueso (enfermedad con cambios anormales del hueso). Si no está seguro, consulte a su médico.
- si ha recibido radioterapia que haya podido afectar a sus huesos.
- si está embarazada o en la lactancia.
Advertencias y precauciones
Livogiva puede causar un aumento de la cantidad de calcio en su sangre u orina.
Consulte a su médico o farmacéutico antes de empezar a usar o mientras esté usando Livogiva:
- si usted tiene continuamente náuseas, vómitos, estreñimiento, baja energía o debilidad muscular dígaselo a su médico. Estos pueden ser síntomas de que hay demasiado calcio en su sangre.
- si usted sufre de piedras en el riñón o presenta una historia previa de piedras en el riñón.
- si usted sufre de problemas de riñón (insuficiencia renal moderada) debe decírselo a su médico.
Algunos pacientes, tras las primeras dosis, sufren mareos o aumento de la frecuencia cardiaca. Para las primeras dosis, utilice Livogiva en un lugar donde pueda sentarse o tumbarse inmediatamente si se marea.
El tiempo de tratamiento recomendado de 24 meses no debe ser excedido. Livogiva no debe utilizarse en adultos en crecimiento.
Niños y adolescentes
Livogiva no debe utilizarse en niños y adolescentes (menores de 18 años).
Otros medicamentos y Livogiva
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, porque ocasionalmente se pueden producir interacciones (p. ej. digoxina/digitálicos, un medicamento empleado para tratar enfermedades cardiacas).
Embarazo y lactancia
No utilice Livogiva si está embarazada o en periodo de lactancia. Si usted es una mujer en edad fértil, debe utilizar métodos anticonceptivos eficaces durante el tratamiento con Livogiva. Si se queda embarazada, debe interrumpirse el tratamiento con Livogiva. Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
Algunos pacientes pueden sentir mareos después de la inyección de Livogiva. Si usted siente mareo no debe conducir o usar máquinas hasta que se encuentre mejor.
Livogiva contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por dosis; esto es, esencialmente «exento de sodio».
3. Cómo usar Livogiva
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es de 20 microgramos administrados una vez al día mediante una inyección debajo de la piel (inyección subcutánea) en el muslo o en el abdomen. Para ayudarle a recordar usar su medicamento, inyéctese sobre la misma hora cada día.
Inyéctese Livogiva cada día durante tanto tiempo como su médico se lo prescriba. La duración total del tratamiento con Livogiva no debe exceder 24 meses. Usted no debe recibir más de un ciclo de 24 meses de tratamiento con Livogiva a lo largo de su vida.
Su médico puede recomendarle usar Livogiva con calcio y vitamina D. Su médico le indicará cuánto debe tomar cada día.
Consulte el Manual del Usuario que está incluido en el estuche con las instrucciones sobre cómo utilizar la pluma Livogiva.
No se incluyen agujas con la pluma. Se pueden utilizar agujas para pluma del calibre 29-31 (diámetro: 0,25-0,33 mm).
La inyección de Livogiva se debe realizar poco después de sacar la pluma de la nevera, tal y como se indica en el Manual de Usuario. Vuelva a guardar la pluma en la nevera inmediatamente después de utilizarla. Debe utilizar una aguja nueva para cada inyección y tirarla después de cada uso. No guarde la pluma con la aguja puesta. Nunca comparta con otros su pluma de Livogiva.
Livogiva puede ser utilizado con o sin alimentos.
Si usa más Livogiva del que debe
Si por error se ha administrado más cantidad de Livogiva de la prescrita, consulte a su médico o farmacéutico.
Los efectos que podrían esperarse de una sobredosis incluyen náuseas, vómitos, mareos y dolor de cabeza.
Si olvida o no puede inyectarse Livogiva a la hora habitual, hágalo tan pronto como pueda ese mismo día. No se administre una dosis doble para compensar las dosis olvidadas. No se inyecte más de una vez en el mismo día. No intente compensar la dosis olvidada.
Si interrumpe el tratamiento con Livogiva
Si está pensando interrumpir el tratamiento con Livogiva, por favor consulte con su médico. Su médico le aconsejará y decidirá sobre cuánto tiempo debe ser tratado con Livogiva.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos más frecuentes son dolor en las extremidades (muy frecuentes, pueden afectar a más de 1 de cada 10 pacientes), malestar, dolor de cabeza y mareo (frecuentes). Si se marea después de una inyección, siéntese o túmbese hasta que se encuentre mejor. En caso de no mejorar, consulte a su médico antes de continuar con el tratamiento. Se han notificado casos de desmayo asociados al uso de teriparatida. Si experimenta molestias como enrojecimiento de la piel, dolor, hinchazón, picor, hematomas o ligero sangrado alrededor de la zona de inyección (frecuente), éstas deberían desaparecer en unos días o semanas. Si no es así, dígaselo a su médico tan pronto como sea posible.
Algunos pacientes, pueden haber experimentado reacciones alérgicas justo después de la inyección, que consisten en dificultad para respirar, hinchazón de la cara, erupción cutánea y dolor en el pecho (frecuencia rara). En raras ocasiones, pueden producirse reacciones alérgicas graves y potencialmente mortales, incluyendo anafilaxia.
Otros efectos adversos son:
Frecuentes: pueden afectar hasta 1 de cada 10 pacientes
- aumento de los niveles de colesterol en sangre
- depresión
- dolor neurálgico en la pierna
- sensación de desvanecimiento
- palpitaciones irregulares
- dificultad para respirar
- aumento de la sudoración
- calambres musculares
- pérdida de energía
- cansancio
- dolor de pecho
- tensión arterial baja
- acidez de estómago (dolor o sensación de ardor justo debajo del esternón)
- vómitos
- hernia del tubo que lleva la comida hasta su estómago
- hemoglobina baja o bajo recuento de glóbulos rojos (anemia)
Poco frecuentes: pueden afectar hasta 1 de cada 100 pacientes
- aumento de la frecuencia cardiaca
- sonido anormal del corazón
- falta de aliento
- hemorroides (almorranas)
- pérdida accidental o escape de orina
- aumento de la necesidad de orinar
- aumento de peso
- piedras en el riñón
- dolor en los músculos y en las articulaciones. Algunos pacientes han experimentado calambres en la espalda graves o dolor y tuvieron que ser hospitalizados.
- aumento en los niveles de calcio en sangre
- aumento de los niveles de ácido úrico en sangre
- aumento en los niveles de una enzima llamada fosfatasa alcalina.
Raros: pueden afectar hasta 1 de cada 1.000 pacientes
- reducción de la función del riñón, incluyendo insuficiencia renal
- hinchazón, principalmente en las manos, pies y piernas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Livogiva
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y la pluma después de CAD y EXP respectivamente. La fecha de caducidad es el último día del mes que se indica.
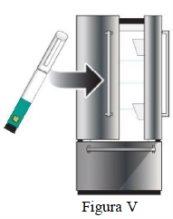
Livogiva debe conservarse siempre en nevera (entre 2°C y 8°C). Puede utilizar Livogiva durante 28 días después de realizar la primera inyección mientras la pluma se conserve en nevera (entre 2ºC y 8ºC).
Evite colocar las plumas cerca del congelador de la nevera para prevenir su congelación. No use Livogiva si está o ha estado congelado.
Cada pluma debe desecharse de forma adecuada después de 28 días, aunque no esté vacía del todo.
Livogiva contiene una solución transparente e incolora. No utilice Livogiva si tiene partículas sólidas o si la solución está turbia o presenta color.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Livogiva
- El principio activo es teriparatida. Cada mililitro de solución inyectable contiene 250 microgramos de teriparatida. Cada pluma precargada de 2,7 ml contiene 675 microgramos de teriparatida (equivalente a 250 microgramos por mililitro).
- Los demás componentes son ácido acético glacial, acetato de sodio trihidrato, manitol, metacresol y agua para preparaciones inyectables. Ver sección 2.
Aspecto del producto y contenido del envase
Livogiva es una solución transparente e incolora. Se presenta en un cartucho incluido en una pluma precargada desechable. Cada pluma contiene 2,7 ml de solución suficiente para 28 dosis. Livogiva está disponible en envases que contienen una o tres plumas precargadas.
Puede que solamente estén disponibles algunos tamaños de envases.
Titular de la autorización de comercialización
Theramex Ireland Limited
3rd Floor Kilmore House, Park Lane, Spencer Dock
DO1 YE64 Dublin 1
Irlanda
Responsable de la fabricación
Eurofins PROXY Laboratories (PRX)
Archimedesweg 25 2333 CM Leiden
Países Bajos
Fecha de la última revisión de este prospecto
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu/.
MANUAL DE INSTRUCCIONES
Livogiva 20 microgramos/80 microlitros solución inyectable en pluma precargada
INFORMACIÓN IMPORTANTE
NOcomience la administración hasta que no haya leído detenidamente el prospecto y este manual de instrucciones contenidos en la caja de Livogiva. Siempre que utilice la pluma Livogiva, siga las instrucciones cuidadosamente.
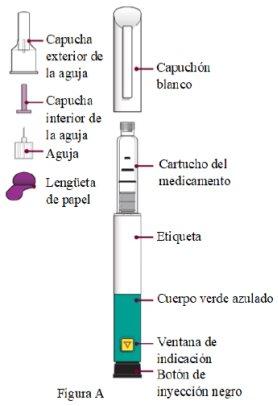
Pluma Livogiva y sus partes Se pueden emplear agujas para pluma del calibre 29-31 (diámetro: 0,25-0,33 mm). Las agujas no están incluidas. |
|
Instrucciones de uso
Preparación de la inyección
Paso 1 Prepare el lugar de inyección y retire el capuchón blanco. |
|
|
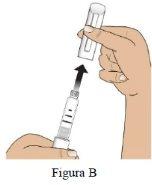
Paso 2 Compruebe la pluma, la etiqueta de la pluma y el medicamento |
NOuse la pluma Livogiva si está deteriorada.
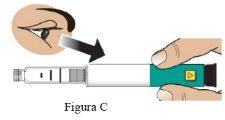
Si la pluma no contiene el medicamento correcto o el medicamento ha caducado, NOlo use (Figura C).
Si el medicamento está turbio, tiene color o contiene partículas en suspensión, NOlo use (Figura C). |
|
Paso 3 Coloque una aguja nueva |
|
|
|
| |
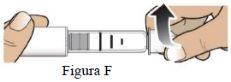
Enrosque la aguja en el sentido de las agujas del reloj hasta que quede perfectamente fijada (Figura F). Noapriete la aguja más de lo necesario. |
|
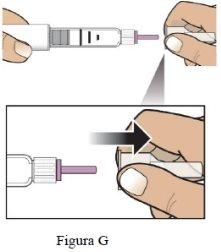
Paso 4 Retire la capucha exterior de la aguja | Retire la capucha exterior grande de la guja (Figura G) y consérvela para más tarde(ver Paso 9). |
|
Paso 5 Ajuste la dosis | Tiredel botón de inyección de color negro hasta que se detenga(Figura H). |
|
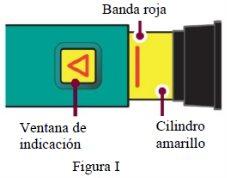
Asegúresede que se ve la banda roja. Además, la ventana de indicación mostrará una flecha que apunta hacia el extremo de la aguja de la pluma (Figura I). |
| |
Resolución de problemas al ajustar la dosis Si la pluma no puede ajustarse completamente o no puede tirar del botón de inyección de color negro, consulte el apartado Resolución del problema E. |
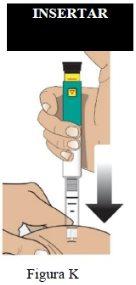
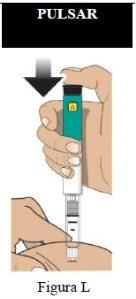
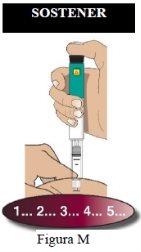
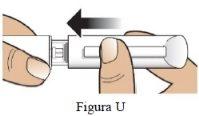
Administración de la inyección
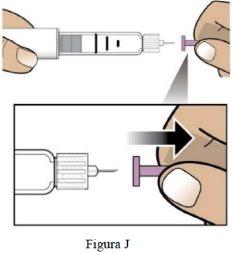
Paso 6 Retire el protector interior de la aguja. | Retireel protector interior pequeño de la aguja y deséchelo (Figura J). La aguja quedará expuesta. |
|
Paso 7 Inyecte la dosis |
|
|
|
| |
|
| |
|
|
Después de la inyección
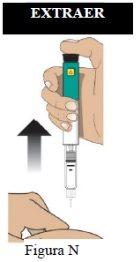
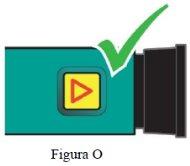
Paso 8 Confirme la dosis. | Asegúresede que el botón de inyección de color negro ha sido introducido hasta el final. La ventana de indicación mostrará una flecha que apunta HACIA el botón de color negro. Si no se muestra el cilindro de color amarillo, habrá finalizado correctamente los pasos de la inyección (Figura O). Importante NOdebe ver ninguna parte del cilindro amarillo. En caso contrario y si ya se ha inyectado el medicamento, NOvuelva a inyectarse una segunda vez en el mismo día. En su lugar, DEBE reajustar la pluma. Consulte el apartado Resolución del problema A. |
|
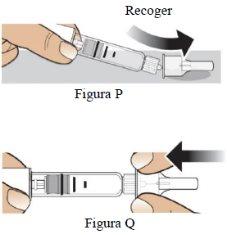
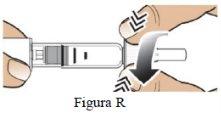
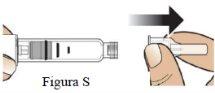
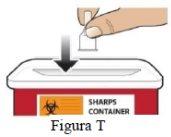
Paso 9 Retire la aguja y deséchela. |
|
|
|
| |
Retire la aguja (Figura S). |
| |
NO reutilice la aguja. |
| |
Eliminación de las agujas Si desea más información acerca del modo adecuado de eliminación de la aguja, consulte el apartado Información referente a la eliminación de la aguja. |
Paso 10 Cubra la pluma de nuevo y consérvela. |
|
|
NOguarde la pluma con una aguja puesta. |
|
Resolución de problemas | |
Problema | Solución |
| Para reajustar la pluma Livogiva, siga los pasos siguientes:
Puede evitar este problema utilizando siempre una aguja NUEVA en cada inyección y presionando el botón de inyección de color negro hasta el final y contando hasta 5 despacio. |
| La pluma Livogiva está diseñada para inyectar la dosis completa cada vez que se utiliza siguiendo las instrucciones del apartado Instrucciones de Uso. El botón de inyección de color negro tiene que estar introducido hasta el final para confirmar que la pluma Livogiva ha inyectado la dosis completa del medicamento. Recuerde utilizar una aguja nueva cada vez que se inyecte para asegurarse de que su pluma Livogiva funciona correctamente. |
| Una pequeña burbuja de aire no afectará a su dosis ni le hará daño. Puede seguir con la administración de su dosis de la forma habitual. |
|
Consulte el paso 9 «Retire la aguja y deséchela». |
| Cambie a una nueva pluma Livogiva para administrarse la dosis tal como le ha indicado su médico o farmacéutico. Cuando cuesta tirar del botón de inyección negro, significa que ya no hay suficiente medicamento en la pluma Livogiva para otra dosis. Es posible que todavía pueda ver algo de medicamento en el cartucho. |
Limpieza y conservación |
Limpieza de la pluma Livogiva
Conservación de la pluma Livogiva
Si se ha dejado la pluma Livogiva fuera de la nevera, no la deseche. Vuelva a meterla en la nevera y póngase en contacto con su médico o farmacéutico. |
Información referente a la eliminación de la aguja |
Eliminación de las agujas de la pluma y la pluma Livogiva
|
Otros puntos de interés |
|
Este manual del usuario ha sido revisado en:
- País de registro
- Precio medio en farmacia252.16 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LIVOGIVA 20 MICROGRAMOS/80 MICROLITROS SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 250 microgramos/mlPrincipio activo: teriparatideFabricante: Gp Pharm S.A.Requiere recetaForma farmacéutica: INYECTABLE, 250 µg/mlPrincipio activo: teriparatideFabricante: Eli Lilly Nederland B.V.Requiere recetaForma farmacéutica: INYECTABLE, 20/80 µg/mlPrincipio activo: teriparatideFabricante: Stada Arzneimittel AgRequiere receta
Médicos online para LIVOGIVA 20 MICROGRAMOS/80 MICROLITROS SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LIVOGIVA 20 MICROGRAMOS/80 MICROLITROS SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes