
MONOPROST 50 MICROGRAMOS/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS


Cómo usar MONOPROST 50 MICROGRAMOS/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Monoprost 50 microgramos/ml colirio en solución en envase unidosis
Latanoprost
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Monoprost y para qué se utiliza
- Qué necesita saber antes de empezar a usar Monoprost
- Cómo usar Monoprost
- Posibles efectos adversos
- Conservación de Monoprost
- Contenido del envase e información adicional
1. Qué es Monoprost y para qué se utiliza
Monoprost pertenece al grupo de medicamentos conocidos como prostaglandinas. Actúa aumentando el flujo natural de líquido desde el interior del ojo al torrente sanguíneo.
Monoprost se utiliza para tratar unas enfermedades conocidas como glaucoma de ángulo abierto e hipertensión ocularen adultos. Ambas enfermedades están relacionadas con un aumento de la presión dentro del ojo, lo que puede llegar a afectar a la visión.
2. Qué necesita saber antes de empezar a usar Monoprost
No use Monoprost
- Si es alérgico (hipersensible) a latanoprost o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico,farmacéutico o enfermero antes de empezar a usar Monoprost si considera que alguna de las siguientes situaciones le afecta:
- Si ha sufrido o va a sufrir una intervención quirúrgica ocular (incluyendo una operación de cataratas).
- Si padece problemas en los ojos (tales como dolor en el ojo, irritación o inflamación, visión borrosa).
- Si padece asma grave o el asma no está bien controlado.
- Si utiliza lentes de contacto. Pueden seguir utilizando Monoprost, pero han de seguir las instrucciones que se incluyen en la sección 3 para usuarios de lentes de contacto.
- Si ha sufrido o está sufriendo una infección vírica del ojo causada por el virus del herpes simple (VHS).
Niños
No se ha investigado Monoprost en los niños (menores de 18 años).
Uso de Monoprost con otros medicamentos
Monoprost puede tener interacciones con otros medicamentos. Informe a su médico o farmacéutico que está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazoy lactancia
No utilice Monoprostsi está embarazada o en periodo de lactancia.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Al utilizar Monoprost puede aparecer visión borrosa durante un periodo de tiempo breve. Si esto le sucede, no conduzcani utilice herramientas o máquinas hasta que su visión vuelva a ser clara de nuevo.
Información importante sobre alguno de los componentes de Monoprost.
Monoprost contienehidroxiestearato de macrogolglicerol (derivado del aceite dericino) , por lo que puede causar reacciones alérgicas.
3. Cómo usar Monoprost
Dosis habitual
- Siga exactamente las instrucciones de administración de Monoprost indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
- La dosis habitual para adultos (incluyendo ancianos), es de una gota en el ojo o en los ojos afectados una vez al día. Es preferible que se administre por la noche.
- No utilice Monoprost más de una vez al día, ya que la eficacia del tratamiento puede disminuir si se administra con mayor frecuencia.
- Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico. En caso de duda, pregunte a su médico, farmacéutico o enfermero.
Usuarios de lentes de contacto
Si usted utiliza lentes de contacto, debe quitárselas antes de utilizar Monoprost. Después de la aplicación de Monoprost, debe esperar 15 minutos antes de volver a ponerse las lentes de contacto.
Instrucciones de uso
El colirio se suministra en envases unidosis. La solución de un envase unidosis de Monoprost debe utilizarse inmediatamente tras su primera apertura para tratar el(los) ojo(s) afectado(s). Dado que no se puede mantener la esterilidad tras la apertura de cada envase unidosis, antes de cada uso debe abrirse un nuevo envase, el cual debe desecharse inmediatamente tras la administración.
Para utilizar el colirio, por favor siga estas instrucciones:
- Lávese las manos y siéntese o permanezca cómodamente de pie.
- Abra el sobre que contiene los envases unidosis. Anote la fecha de primera apertura en el sobre.
- Separe un envase unidosis de la tira.
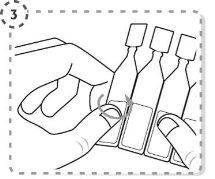
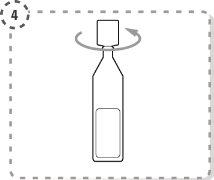
- Gire la punta del envase unidosis tal y como se muestra. No toque la punta después de abrir el envase.
- Utilizando el dedo separe con suavidad el párpado inferior del ojo afectado.
- Coloque la punta del envase unidosis cerca del ojo, pero sin llegar a tocarlo.
- Presione con suavidad el envase unidosis de forma que caiga solamente una gota en el ojo y luego retire el dedo del párpado inferior.
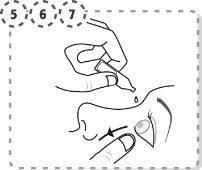
- Presione con el dedo el extremo del ojo afectado, en la parte cercana a la nariz. Ejerza la presión durante 1 minuto, manteniendo su ojo cerrado.
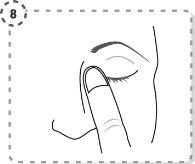
- Repita la operación en el otro ojo, si su médico se lo ha indicado. Cada envase unidosis contiene cantidad suficiente para los dos ojos.
- Después de utilizarlo, deseche el envase unidosis. No lo guarde para utilizarlo otra vez. Como no se puede garantizar la esterilidad del envase unidosis tras su apertura, se debe abrir un nuevo envase antes de cada uso.
Si usa Monoprost con otros colirios
Espere al menos 5 minutos entre la aplicación de Monoprost y la administración de otros colirios.
Si usa más Monoprost del que debe
Si se ha aplicado más gotas en el ojo de las que debe, puede sentir una ligera irritación en el ojo y también puede que los ojos se pongan rojos y que lloren; esta situación debería desaparecer, pero si le preocupa, contacte con su médico.
En caso de ingestión accidental de Monoprost consulte a su médico lo antes posible
Si olvidó usar Monoprost
Continúe con la administración de la siguiente dosis de la forma habitual. No debe aplicarse una gota adicional en el ojo para compensar la dosis olvidada. Si tiene dudas consulte con su médico o farmacéutico.
Si interrumpe el tratamiento con Monoprost
Si desea dejar de utilizar Monoprost, debe consultar con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las siguientes reacciones adversas son conocidas con el uso de Monoprost:
Muy frecuentes: puedenafectar a más de 1 de cada 10 pacientes.
- Cambio gradual en el color de los ojos por el incremento de la cantidad de pigmento de color marrón de la parte coloreada del ojo conocida como iris.
- Si tiene ojos de color mixto (azul-marrón, gris-marrón, amarillo-marrón o verde-marrón) es más probable que vea este cambio que si sus ojos son de un solo color (azul, gris, verde o marrón).
- El cambio en el color de los ojos puede tardar años en desarrollarse, aunque puede apreciarse normalmente a los 8 meses de tratamiento.
- El cambio de color puede ser permanente y puede ser más llamativo si Monoprost se utiliza únicamente en un ojo.
- El cambio en el color del ojo no parece estar asociado a la aparición de ningún problema.
- El cambio en el color del ojo no progresa una vez que se ha suspendido el tratamiento con Monoprost.
- Enrojecimiento del ojo.
- Irritación ocular (sensación de escozor, sensación de arenilla en el ojo, picor, dolor y sensación de cuerpo extraño en el ojo).
- Cambio gradual en las pestañas del ojo tratado y del vello fino que hay alrededor del ojo tratado, observado en la mayoría de los pacientes de origen japonés. Estos cambios incluyen un aumento del color (oscurecimiento), alargamiento, engrosamiento y aumento del número de pestañas.
Frecuentes: puedenafectar hasta 1 de cada 10 pacientes
- Irritación o erosión en la superficie del ojo, inflamación del párpado (blefaritis) y dolor en el ojo, y sensibilidad a la luz (fotofobia), conjuntivitis.
Poco frecuentes: puedenafectar hasta 1 de cada 100 pacientes
- Hinchazón de los párpados, ojo seco, inflamación o irritación de la superficie del ojo (queratitis), visión borrosa, inflamación de la parte coloreada del ojo (uveítis), hinchazón de la retina (edema macular).
- Erupción de la piel.
- Dolor de pecho (angina), sentir el ritmo cardiaco (palpitaciones).
- Asma, dificultad en la respiración (disnea).
- Dolor de pecho.
- Dolor de cabeza, mareo.
- Dolor muscular, dolor articular.
- Náuseas, vómitos.
Raros: puedenafectar hasta 1 de cada 1.000 pacientes
- Inflamación del iris (iritis), síntomas de hinchazón o lesión/daño en la superficie del ojo, hinchazón alrededor del ojo (edema periorbitario), pestañas desviadas o hilera adicional de pestañas, acumulación de líquido en la parte coloreada del ojo (quiste del iris).
- Reacciones en la piel de los párpados, oscurecimiento de la piel de los párpados.
- Empeoramiento del asma, .
- Picor intenso en la piel.
- Desarrollo de una infección vírica del ojo causada por el virus del herpes simple (VHS).
Muy raros: puedenafectar hasta 1 de cada 10.000 pacientes
- Agravamiento de la angina en pacientes que también tienen problemas cardiacos.
- Aspecto de hundimiento del ojo (profundización del sulcus del ojo).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https//: notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Monoprost
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, sobre y en el envase unidosis. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 25°C.
Tras la primera apertura del sobre: utilizar los envases unidosis dentro de los 10 días siguientes.
Tras la primera apertura del envase unidosis: utilizar inmediatamente y desechar el envase unidosis después de utilizarlo.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deMonoprost
El principio activo es latanoprost.
1 ml de colirio contiene 50 microgramos de latanoprost.
Los demás componentes (excipientes) son: hidroxiestearato de macrogolglicerol 40, sorbitol, carbómero 974P, macrogol 4000, edetato de disodio, hidróxido de sodio (para ajuste del pH), agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Este medicamento se presenta como un colirio en solución, en envases unidosis. La solución es ligeramente amarilla y opalescente, sin conservantes, contenida en envases unidosis, presentados en el interior de un sobre con 5 o 10 unidades. Cada envase unidosis contiene 0,2 ml de colirio en solución.
Las cajas contienen 5 (1 x 5), 10 (2 x 5), 10 (1X10), 30 (6 x 5), 30 (3X10), 90 (18 x 5) o 90 (9X10) envases unidosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
LABORATOIRES THEA
12 RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCIA
Responsable de la fabricación
EXCELVISION
27, rue de la Lombardière
ZI la Lombardière
07100 ANNONAY
FRANCIA
O
LABORATOIRES THEA
12 RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCIA
O
LABORATOIRE UNITHER
1 rue de l’Arquerie 50200 Coutances
FRANCIA
O
FAREVA Mirabel
Route de Marsat
Riom
63693 Clermont-Ferrand Cedex 9
FRANCIA
Representante local
LABORATORIOS THEA, S.A.
C/ Enric Granados, nº 86-88, 2ª planta
08008 – Barcelona
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania, Bélgica, Bulgaria, Chipre, Dinamarca, España, Finlandia, Francia, Grecia, Holanda, Islandia, Italia, Letonia, Luxemburgo, Noruega, Polonia, Portugal, Suecia
Monoprost
Irlanda Monopost Unidose
Austria, Eslovenia, Lituania, República Checa, República Eslovaca, Reino Unido, Rumania
Monopost
Estonia…………………………………………………………………………………Monopro
Fecha de la última revisión de este prospecto: Mayo 2025
- País de registro
- Precio medio en farmacia15.61 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MONOPROST 50 MICROGRAMOS/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 50 microgramos/mlPrincipio activo: LatanoprostFabricante: Santen OyRequiere recetaForma farmacéutica: COLIRIO, 50 µg/mlPrincipio activo: LatanoprostFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: COLIRIO, 50 MG/MLPrincipio activo: LatanoprostFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para MONOPROST 50 MICROGRAMOS/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MONOPROST 50 MICROGRAMOS/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















