INTESTIFALK 2 mg/DOSIS ESPUMA RECTAL

Cómo usar INTESTIFALK 2 mg/DOSIS ESPUMA RECTAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el usuario
Intestifalk 2 mg/dosis espuma rectal
budesónida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico,incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Intestifalk espuma rectal y para qué se utiliza
- Qué necesita saber antes de empezar a usar Intestifalk espuma rectal
- Cómo usar Intestifalk espuma rectal
- Posibles efectos adversos
- Conservación de Intestifalk espuma rectal
Contenido del envase e información adicional
1. Qué es Intestifalk espuma rectal y para qué se utiliza
Intestifalk espuma rectal contiene el principio activo budesónida, un antiinflamatorio de tipo esteroide de acción local para tratar enfermedades inflamatorias del intestino.
Intestifalk espuma rectal está indicado para el tratamiento de:
una enfermedad inflamatoria del recto y del intestino grueso (colon sigmoide) llamada por los médicos colitis ulcerosa.
2. Qué necesita saber antes de empezar a usarIntestifalk espuma rectal
No use Intestifalk espuma rectal
- Si es alérgico a la budesónida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si padece una enfermedad hepática grave(cirrosis hepática).
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Intestifalk espuma rectal si padece::
- tuberculosis.
- tensión sanguínea alta.
- diabetes o algún miembro de su familia ha sido diagnosticado de diabetes.
- fragilidad de los huesos (osteoporosis).
- úlceras en el estómago o primera parte del intestino delgado (úlcera péptica).
- presión aumentada en el ojo (glaucoma) o problemas en los ojos como opacificación del cristalino (cataratas) o si a algún miembro de su familia se le ha diagnosticado glaucoma.
- problemas hepáticos graves.
Pueden aparecer los efectos típicos de las preparaciones de cortisona pudiendo afectar a todas las partes del cuerpo, particularmente si usted usa Intestifalk espuma rectal a dosis elevadas y durante periodos de tiempo prolongados (ver sección 4. Posibles efectos adversos).
Precauciones adicionales durante el tratamiento con Intestifalk espuma rectal:
- Informe a su médico si tiene una infección. Los síntomas de algunas infecciones pueden ser atípicos o menos marcados.
- Manténgase alejado de personas que tengan varicela o herpes zoster (culebrilla) si no los ha padecido antes. Pueden afectarle gravemente. Si entra en contacto con varicela o herpes, vea a su médico inmediatamente.
- Informe a su médico si no ha padecido sarampión.
- Si durante el tratamiento con este medicamento tiene que recibir alguna vacuna, informe antes a su médico.
- Informe a su médico que está utilizando Intestifalk espuma rectal en caso de intervención quirúrgica programada.
- Si ha estado siendo tratado con una preparación de cortisona más potente antes de comenzar con el tratamiento de Intestifalk espuma rectal, sus síntomas pueden reaparecer al cambiar el medicamento. Si esto ocurre, informe a su médico.
- Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Uso de Intestifalk espuma rectal con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente,o podría tener que utilizar otros medicamentos .En particular :
- glucósidos cardiotónicostales como digoxina (medicamentos utilizados para tratar alteraciones cardíacas)
- diuréticos(medicamentos utilizados para tratar el exceso de fluido en su cuerpo)
- ketoconazol o itraconazol(para tratar infecciones fúngicas)
- antibióticos,medicamentospara tratar infecciones (tales como claritromicina)
- carbamazepina(utilizada en el tratamiento de la epilepsia)
- rifampicina(para tratar la tuberculosis)
- estrógenos o anticonceptivos orales
Intestifalk espuma rectal podría alterar los resultados de las pruebas efectuadas por su médico o en un hospital. Informe a su médico de que está usando Intestifalk espuma rectal antes de la realización de cualquier prueba.
Algunos medicamentos pueden aumentar los efectos de Intestifalk espuma rectal, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Uso de Intestifalk espuma rectal con alimentos y bebidas
Nodebe de tomar zumo de pomelodurante su tratamiento conIntestifalk espuma rectal, ya que este, puede modificar sus efectos
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Sólo debe de usar Intestifalk espuma rectal durante el embarazo si el médico se lo indica.
La budesónida pasa en pequeñas cantidades a la leche materna. Si está dando el pecho, sólo debe usar Intestifalk espuma rectal si su médico se lo indica.
Conducción y uso de máquinas
No es de esperar que Intestifalk espuma rectal tenga efectos sobre la capacidad para conducir o utilizar maquinaria.
Intestifalk espuma rectal contiene propilenglicol, alcohol cetílico y alcohol cetoestearílico
Este medicamento contiene 600,3 mg de propilenglicol en cada pulsación de Intestifalk espuma rectal. El propilenglicol puede causar irritación en la piel.
El alcohol cetílico y el alcohol cetoestearílico (componente de la cera emulsificante) pueden producir reacciones locales en la piel (como dermatitis de contacto).
.
3. Cómo usarIntestifalk espuma rectal
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es:
Posología para adultos mayores de 18 años
La dosis normal es de 1 aplicación en spray, una vez al día por la mañana o a la hora de irse a la cama. Los mejores resultados se producen utilizando Intestifalk espuma rectal tras vaciar su intestino.
Uso en niños y adolescentes
Intestifalk espuma rectal no debe de utilizarse en niños menores de 18 años, ya que se dispone de una experiencia muy limitada en este colectivo.
Método de administración
Este medicamento solo se puede utilizar por vía rectal, por lo que se debe introducir por el ano. No está indicado para el uso oral. No ingerir.
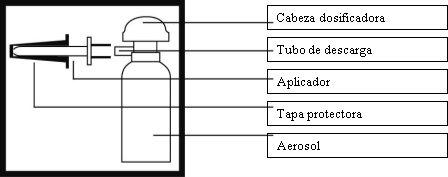
Ilustración del envase aerosol
El aplicador y su tapa protectora van en una moldura especial. Por favor, coja la moldura firmemente y tire del aplicador con fuerza.
Preparación para la utilización de la espuma:
 Conectar el aplicador firmemente al tubo de descarga del envase. Agitar el envase durante aproximadamente 15 segundos para mezclar el contenido.
Conectar el aplicador firmemente al tubo de descarga del envase. Agitar el envase durante aproximadamente 15 segundos para mezclar el contenido.

 Antes de utilizar por primer vez, retirar el cierre de seguridad (solapa de plástico) de la cabeza dosificadora.
Antes de utilizar por primer vez, retirar el cierre de seguridad (solapa de plástico) de la cabeza dosificadora.
 Girar la cabeza del envase hasta que la muesca semicircular situada debajo de la cabeza quede en línea con el aplicador. Ahora el aerosol está listo para utilizar.
Girar la cabeza del envase hasta que la muesca semicircular situada debajo de la cabeza quede en línea con el aplicador. Ahora el aerosol está listo para utilizar.
Utilización de la espuma:
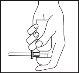 Coloque su dedo índice en la parte superior de la cabeza dosificadora y gire el aerosol hacia abajo. Tenga en cuenta que el aerosol sólo trabaja adecuadamente cuando se sostiene con la cabeza dosificadora hacia abajo lo más verticalmente posible.
Coloque su dedo índice en la parte superior de la cabeza dosificadora y gire el aerosol hacia abajo. Tenga en cuenta que el aerosol sólo trabaja adecuadamente cuando se sostiene con la cabeza dosificadora hacia abajo lo más verticalmente posible.
| Coloque un pie sobre una silla o taburete y recuéstese de lado con la parte inferior de la pierna estirada y la parte superior de la pierna doblada para mantener el equilibrio. Inserte el aplicador en su recto tanto como le sea posible. Presione completamente la cabeza dosificadora una vez y, a continuación, libérela muy lentamente; la espuma sale del aerosol cuando libera la cabeza dosificadora. Mantenga el aplicador en su sitio de 10 a 15 segundos antes de retirarlo. Esto garantiza que se administra la dosis completa y que no se derrama nada de espuma. |
 Tras la administración de la espuma, separe el aplicador y deséchelo con su basura doméstica utilizando una de las bolsas de plástico que se acompañan. Para la siguiente aplicación utilice un nuevo aplicador.
Tras la administración de la espuma, separe el aplicador y deséchelo con su basura doméstica utilizando una de las bolsas de plástico que se acompañan. Para la siguiente aplicación utilice un nuevo aplicador.
Para evitar la pérdida inadvertida de espuma entre aplicaciones, gire la cabeza dosificadora de forma que la muesca semicircular esté en dirección opuesta al tubo de descarga.
- Lávese las manos y trate de no evacuar su intestino hasta la mañana siguiente.
- En el caso de que vaya a un hospital o vaya a ver a otro médico o al dentista, dígale que esta utilizando este medicamento.
Duración del tratamiento
La duración del tratamiento depende de la naturaleza de su enfermedad.
Su médico decidirá durante cuánto tiempo tiene que seguir usando la medicación.
Los episodios agudos leves de la enfermedad inflamatoria intestinal (colitis ulcerosa) generalmente remiten tras 6-8 semanas.
Si tiene la impresión de que el efecto de Intestifalk espuma rectal es demasiado fuerte o demasiado débil, consulte con su médico.
Si usa más Intestifalk espuma rectal del que debe
Si ha usado demasiado medicamento en una ocasión, utilice la siguiente dosis como le han prescrito. No use una cantidad menor. Si tiene alguna duda, póngase en contacto con su médico para que él o ella decida qué debe hacer, de ser posible, lleve consigo la caja y el prospecto.

Si olvidó usar Intestifalk espuma rectal
Si se olvidó de una dosis, continúe el tratamiento en la dosis prescrita. No use una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Intestifalk espuma rectal
Hable con su médico si quiere interrumpir o concluir su tratamiento antes. Es importante que no deje de usar su medicamento repentinamente ya que esto podría enfermarle. Continúe usando su medicamento hasta que su médico se lo indique, aun cuando empiece a sentirse mejor.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si usted tiene alguno de los siguientes síntomas después de usar este medicamento, deberá contactar con su médico inmediatamente:
- Infección
- Dolor de cabeza
- Cambios en la conducta como depresión, irritabilidad, euforia, inquietud, ansiedad o agresividad.
Se ha notificado también de los siguientes efectos adversos:
Frecuentes: pueden afectar hasta a 1 de cada 10 personas
- Quemazón o dolor en el recto
- Síndrome de Cushing-por ejemplo cara de luna llena, aumento del peso corporal, reducción de la tolerancia a la glucosa, aumento del azúcar en sangre, presión sanguínea elevada, retención de líquidos en los tejidos (por ejemplo: piernas hinchadas), incremento en la excreción de potasio (hipocalemia), períodos irregulares en las mujeres, hirsutismo no deseado en mujeres, impotencia, hallazgos anómalos en el laboratorio (función adrenal reducida), formación de estrías rojas en la piel (marcas de estiramiento), acné
- Indigestión, estómago irritable (dispepsia)
- Aumento del riesgo de infección
- Dolor muscular y de las articulaciones, debilidad muscular, calambres musculares
- Fragilidad de los huesos (osteoporosis)
- Dolor de cabeza
- Cambios de humor, tales como depresión, irritación o euforia
- Sarpullido debido a reacciones de hipersensibilidad, manchas rojas debidas a hemorragias en la piel, retraso en la curación de las heridas, reacciones locales de la piel tales como dermatitis de contacto
Poco frecuentes: pueden afectar hasta a 1 de cada 100 personas
- Aumento de apetito
- Alteraciones en la sangre (aumento de la velocidad de sedimentación globular, aumento del número de glóbulos blancos)
- Náuseas, dolor abdominal, gases, acorchamiento u hormigueo en el abdomen, fisura anal, úlceras en la boca, necesidad urgente de vaciar el intestino, sangrado rectal.
- Úlceras en el estómago o en el intestino delgado
- Cambios en los parámetros de la función hepática
- Alteraciones en la función pancreática, variaciones de las hormonas suprarrenales
- Infecciones en el tracto urinario
- Mareo, alteraciones del olfato
- Insomnio, inquietud con aumento de la actividad física, ansiedad
- Aumento de sudoración, debilidad
Raros: pueden afectar hasta a 1 de cada 1.000 personas
- Visión borrosa .
- Inflamación del páncreas
- Pérdida de hueso debida a mala circulación de la sangre (osteonecrosis)
- Agresividad
- Moratones
Muy raros: pueden afectar hasta a 1 de cada 10.000 personas
- Retraso del crecimiento en niños
- Estreñimiento
- Incremento de la presión cerebral, posiblemente con incremento de la presión ocular
(inflamación del disco óptico) en adolescentes
- Aumento del riesgo de trombosis, inflamación de los vasos sanguíneos (asociado a la
retirada de cortisona tras un tratamiento a largo plazo)
- Cansancio, sensación de malestar general
Estos efectos adversos son típicos de medicamentos esteroideos y la mayoría de ellos también son previsibles para los tratamientos con otros esteroides. Pueden aparecer dependiendo de la dosis, duración del tratamiento, de si se ha seguido o se está siguiendo un tratamiento con otras preparaciones de cortisona y de la propia sensibilidad personal.
Algunas de las reacciones adversas se notificaron sólo tras una administración oral de budesónida a largo plazo.
Debido a su acción local, el riesgo de reacciones adversas a Intestifalk 2 mg espuma rectal es generalmente menor que el tratamiento con glucocorticoesteroides de acción sistémica (su acción se extiende sobre todo el cuerpo).
Si ha recibido tratamiento con una preparación de cortisona más potente antes de iniciar el tratamiento con Intestifalk espuma rectal, sus síntomas pueden reaparecer al cambiar el medicamento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Intestifalk espuma rectal
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el envase presurizado. La fecha de caducidad es el último día del mes que se indica.
El contenido del envase debe utilizarse en 4 semanas una vez abierto.
No conservar a temperatura superior a 25º C.
No refrigerar o congelar.
El envase está presurizado y contiene un propelente inflamable.
No exponer a temperaturas superiores a 50ºC, proteger de la luz solar directa.
No perforar o quemar aun estando vacíos.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medioambiente.
6. Contenido del envase e información adicional
Composición de Intestifalk espuma rectal
El principio activo es la budesónida. Cada dosis contiene 2 mg de budesónida.
Los demás componentes son alcohol cetílico, cera emulsificante, agua purificada, edetato disódico, éter estearílico macrogol, propilenglicol, ácido cítrico monohidrato y n-butano, isobutano y propano como propelentes.
Aspecto del producto y contenido del envase
Intestifalk espuma rectal es una espuma cremosa firme de color blanco a blanco grisáceo, presentándose en un envase presurizado.
Intestifalk espuma rectal está disponible en cajas con 1 envase presurizado, 14 aplicadores y 14 bolsas de plástico o en cajas con 2 envases presurizados, 28 aplicadores y 28 bolsas de plástico para desechar de forma higiénica los aplicadores.
Puede que no todos los envases estén comercializados.
Titular de la autorización de comercialización y responsable de la fabricación
Dr. Falk Pharma GmbH
Leinenweberstr. 5
79108 Freiburg
Alemania
TEL +49 (0) 761 / 1514-0
E-mail: [email protected]
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
España
Dr. Falk Pharma España
Camino de la Zarzuela, 19
28023 Madrid
Este medicamento está autorizado en los estados miembrosdel Espacio Económico Europeocon los siguientes nombres:
Dinamarca, Finlandia, Grecia, Irlanda, Rumania , Suecia y Reino Unido:
Budenofalk.
Austria: Budo-San.
Italia:Intesticort.
España: Intestifalk.
Fecha de la última revisión de este prospecto:Septiembre 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es/
- País de registro
- Precio medio en farmacia80.61 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a INTESTIFALK 2 mg/DOSIS ESPUMA RECTALForma farmacéutica: LIQUIDO RECTAL, 2 mg budesonidaPrincipio activo: BudesonidaFabricante: Tillotts Pharma GmbhRequiere recetaForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 3 mg budesonidaPrincipio activo: BudesonidaFabricante: Tillotts Pharma GmbhRequiere recetaForma farmacéutica: CAPSULA, 3 mg budesonidaPrincipio activo: BudesonidaFabricante: Dr. Falk Pharma GmbhRequiere receta
Médicos online para INTESTIFALK 2 mg/DOSIS ESPUMA RECTAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de INTESTIFALK 2 mg/DOSIS ESPUMA RECTAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes