ENTOCORD 2 MG COMPRIMIDO Y DISOLVENTE PARA SUSPENSION RECTAL

Cómo usar ENTOCORD 2 MG COMPRIMIDO Y DISOLVENTE PARA SUSPENSION RECTAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Entocord 2 mg comprimido y disolvente para suspensión rectal
budesónida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Entocord y para qué se utiliza
- Qué necesita saber antes de empezar a usar Entocord
- Cómo usar Entocord
- Posibles efectos adversos
- Conservación de Entocord
- Contenido del envase e información adicional
1. Qué es Entocord y para qué se utiliza
Entocord (budesónida) pertenece a un grupo de medicamentos denominados glucocorticosteroides (un tipo de cortisona) que son utilizados para reducir la inflamación.
Entocord se utiliza en el tratamiento de la colitis ulcerosa, enfermedad producida por la inflamación de la pared del intestino, que afecta al recto y al colon sigmoide y descendente.
2. Qué necesita saber antes de empezar a usar Entocord
No use Entocord
Si es alérgico a budesónida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Entocord.
Usted debe informar siempre a su médico de las situaciones descritas a continuación:
- Si presenta otros problemas de salud, tales como enfermedad hepática.
- Si está padeciendo o contrae alguna infección, particularmente varicela o sarampión.
- Si presenta diabetes (incluyendo antecedentes familiares), fragilidad ósea (osteoporosis), úlcera de estómago o presión arterial alta.
- Si padece de alguna enfermedad ocular como glaucoma (incluyendo antecedentes familiares) o cataratas.
- Si sus síntomas empeoran mientras está utilizando Entocord.
No interrumpa el tratamiento con Entocord hasta que su médico se lo diga.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Entocord le ha sido prescrito específicamente para su actual dolencia. No lo utilice para otros problemas a menos que su médico así se lo diga.
Si previamente había sido tratado con comprimidos de “cortisona” (tales como prednisona, prednisolona o metilprednisolona) y su medicación ha sido cambiada a Entocord, temporalmente podrían reaparecer síntomas que podían haberle molestado anteriormente, por ejemplo, erupciones cutáneas, dolor en los músculos y articulaciones. Si alguno de estos síntomas le molesta o aparecen síntomas tales como dolor de cabeza, cansancio, náuseas o vómitos, por favor, contacte con su médico.
Niños y adolescentes
No hay datos a largo plazo de tratamientos en niños y adolescentes, se recomienda controlar regularmente su estatura.
Otros medicamentos y Entocord
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Ciertos medicamentos pueden interaccionar con Entocord; en estos casos, puede resultar necesario cambiar la dosis o interrumpir el tratamiento con alguno de los medicamentos.
Es importante que informe a su médico si toma alguno de los siguientes fármacos: ketoconazol, itraconazol (medicamentos utilizados contra las infecciones causadas por hongos), carbamazepina (antiepiléptico) o en mujeres, estrógenos y algunos anticonceptivos.
Algunos medicamentos pueden aumentar los efectos de Entocord y puede que su médico desee supervisarle cuidadosamente si está tomando estos medicamentos (incluyendo algunos medicamentos para el tratamiento del VIH: ritonavir (y otros inhibidores de la proteasa del VIH, cobicistat).
Las pruebas de diagnóstico para la actividad de las glándulas pituitaria pueden mostrar resultados bajos falsos debido a la supresión de la función adrenal.
Embarazo y lactanciaSi está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, farmacéutico o enfermero antes de utilizar este medicamento.
Conducción y uso de máquinasEntocord no afecta la capacidad para conducir vehículos o manejar maquinaria.
Entocord contiene parahidroxibenzoato de metilo y de propilo
Puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo y de propilo.
Si usted es deportista deberá tener en cuenta que este medicamento contiene un componente que puede establecer un resultado analítico de control del dopaje como positivo.
3. Cómo usar Entocord
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Recuerde usar su medicamento.
Su médico le indicará la duración de su tratamiento con Entocord. No suspenda el tratamiento antes de que su médico se lo diga.
Antes de utilizar Entocord por primera vez es importante que lea las Instrucciones de Uso que se
describen a continuación. Estas instrucciones le indicarán cómo preparar y utilizar Entocord. Siga cuidadosamente las instrucciones.
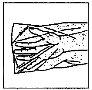
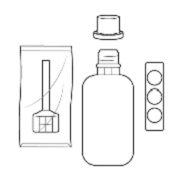
Instrucciones de uso/ manipulación Entocord debe ser administrado por la noche antes de acostarse. Entocord está constituido por: un comprimido, un frasco de disolvente de 115 mL y una cánula rectal envasada individualmente. Para la reconstitución del medicamento, debe disolverse el comprimido en el disolvente antes de su uso. Para la correcta administración de Entocord deben seguirse cuidadosamente las siguientes instrucciones: |
| |
Cómo preparar Entocord | ||
|
| |
|
| |
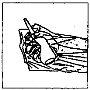
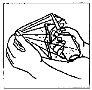
Cómo administrar Entocord Se incluyen en el envase fundas de plástico para proteger su mano durante la administración del medicamento (ver las ilustraciones más adelante).
Adopte una posición cómoda para dormir. Procure retener el Entocord tanto tiempo como sea posible, preferiblemente toda la noche. |
| |
|
|
|
|
|
|
Una vez preparada la suspensión rectal, ésta debe ser administrada inmediatamente. No debe ser guardada en el frasco.
La posología se adapta a cada individuo. Siga cuidadosamente las instrucciones de su médico. Estas instrucciones podrían ser diferentes de la información contenida en este prospecto.
Dosis recomendada para adultos
Se recomienda administrar un Entocord (comprimido + frasco de disolvente de 115 mL) al día (cada noche) durante cuatro semanas. El mejor momento para utilizar Entocord es por la noche, justo antes de acostarse. Así, Entocord permanecerá en su intestino el mayor tiempo posible mientras está durmiendo.
El efecto completo se consigue generalmente en 2-4 semanas. Sin embargo, si sus síntomas no han mejorado después de cuatro semanas de tratamiento, su médico podría prolongar su tratamiento otras 4 semanas.
Entocord debe ser utilizado regularmente según lo prescrito. No olvide administrarse Entocord aunque comience a sentirse mejor.
Pacientes de edad avanzada
La misma posología que para adultos.
Si usa más Entocord del que debe
En caso de sobredosis o ingestión accidental, consultar al Servicio de Información Toxicológica. Teléfono 91 562 04 20
Si olvidó usar Entocord
Si ocasionalmente olvida la administración de alguna dosis de Entocord, no es necesario compensar la dosis que olvidó, simplemente continúe con la siguiente dosis según lo prescrito.
No use una dosis doble para compensar las dosis olvidadas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si usted tiene una reacción alérgica, consulte a su médico inmediatamente.Los signos pueden incluir bultos en la piel (ronchas) o hinchazón de la cara, labios, boca, lengua o garganta. Esto puede hacer que sea difícil respirar.
Otros posibles efectos adversos:
Frecuentes: puede afectar hasta 1 de cada 10 personas
- Dolor de estómago o de intestino, tales como dolores de estómago, flatulencias, diarrea, ardor de estómago y sensación de malestar.
- Calambres.
- Reacciones cutáneas, tales como erupción grumosa y erupción cutánea.
- Cambios en el comportamiento, tales como nerviosismo, insomnio, cambios del estado de ánimo y depresión.
- Palpitaciones.
- Bajos niveles de potasio en sangre.
- Trastornos menstruales.
- Rasgos cushingoides como cara redondeada, acné, ganancia de peso y aparición de hematomas fácilmente.
Poco frecuentes: puede afectar hasta 1 de cada 100 personas
- Movimientos involuntarios o inquietud extrema, posiblemente acompañados de espasmos o sacudidas musculares.
- Ansiedad.
- Temblor.
Raros: puede afectar hasta 1 de cada 1.000 personas
- Alteración en la glándula suprarenal (una glándula pequeña cerca del riñón).
- Agresividad.
- Opacidad del cristalino natural del ojo incluyendo la parte posterior (cataratas).
- Glaucoma (incremento de la presión ocular).
- Visión borrosa.
- Alteración de la coloración de la piel como resultado de una hemorragia interna.
Muy raros: puede afectar hasta 1 de cada 10.000 personas
- Reacción alérgica grave (llamada anafilaxis) que puede causar dificultad para respirar o shock.
- Un retraso en la tasa de crecimiento en niños y adolescentes.
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles
- Reacciones alérgicas que pueden causar hinchazón de la cara, especialmente de los párpados, los labios, la lengua o la garganta (angioedema).
Los medicamentos como Entocord (corticosteroides) pueden afectar a la producción normal de hormonas esteroides en su cuerpo. Los efectos incluyen:
- Cambios en la densidad mineral ósea (adelgazamiento de los huesos).
- Glaucoma (incremento de la presión ocular).
- Disminución de la tasa de crecimiento en niños y adolescentes.
- Alteración en la glándula suprarenal (una glándula pequeña cerca del riñón).
La mayoría de los efectos adversos mencionados en esta lista también se pueden esperar con el tratamiento con otros glucocorticoides.
No se alarme por esta lista de efectos adversos. Puede que no sufra ninguno de ellos. Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico, farmacéutico o enfermero.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Entocord
Mantener este medicamento fuera de la vista y del alcance de los niños. No conservar los comprimidos y los frascos de disolvente a temperatura superior a 30ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deEntocord
- El principio activo es budesónida. Entocord (comprimido y frasco de disolvente de 115 mL) contiene 2 mg de budesónida por 100 mL (0,02 mg/mL).
- Entocord está constituido por: un comprimido dispersable, un frasco de disolvente de 115 mL y una cánula rectal envasada individualmente.
- Cada comprimido contiene: 2,3 mg del principio activo budesónida
- Los demás excipientes son: lactosa, colorante [riboflavina (E101)], crospovidona, sílice coloidal anhidra y estearato de magnesio.
- Cada frasco de disolvente de 115 mL contiene: cloruro de sodio, conservantes, [parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216)] y agua.
Aspecto del producto y contenido del envase
Entocord se presenta en forma de comprimidos dispersables y disolvente para suspensión rectal. Los envases contienen un blíster con 7 comprimidos, 7 frascos de disolvente de 115 mL, 7 cánulas rectales (aplicadores del enema) y 7 fundas de plástico para utilizar durante la aplicación del enema.
Titular de la autorización de comercializacióny responsable de la fabricación
Titular de la autorización de comercialización:
Tillotts Pharma GmbH
Warmbacher Str. 80
79618 Rheinfelden
Alemania
Responsable de la fabricación:
Lusomedicamenta, Sociedade Técnica Farmacêutica, SA
Estrada Consiglieri Pedroso, 69-B
Queluz de Baixo
2730-055 Barcarena
Portugal
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Tillotts Pharma Spain, S.L.U.
Travessera de Gràcia 58, 5º 3ª
08006 Barcelona
España
Fecha de la última revisión de este prospecto:Julio 2023
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia40.31 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ENTOCORD 2 MG COMPRIMIDO Y DISOLVENTE PARA SUSPENSION RECTALForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 3 mg budesonidaPrincipio activo: BudesonidaFabricante: Tillotts Pharma GmbhRequiere recetaForma farmacéutica: SEMISOLIDO RECTAL, 2 MGPrincipio activo: BudesonidaFabricante: Dr. Falk Pharma GmbhRequiere recetaForma farmacéutica: CAPSULA, 3 mg budesonidaPrincipio activo: BudesonidaFabricante: Dr. Falk Pharma GmbhRequiere receta
Médicos online para ENTOCORD 2 MG COMPRIMIDO Y DISOLVENTE PARA SUSPENSION RECTAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ENTOCORD 2 MG COMPRIMIDO Y DISOLVENTE PARA SUSPENSION RECTAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes




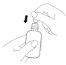 (Fig. 1).
(Fig. 1).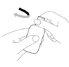 (Fig. 2).
(Fig. 2).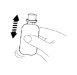 (Fig. 3).
(Fig. 3).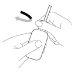 (Fig. 4).
(Fig. 4).