
FENTANILO KALCEKS 50 MICROGRAMOS/ML SOLUCION INYECTABLE EFG

Cómo usar FENTANILO KALCEKS 50 MICROGRAMOS/ML SOLUCION INYECTABLE EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Fentanilo Kalceks 50 microgramos/ml solución inyectable EFG
fentanilo
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fentanilo Kalceks y para qué se utiliza
- Qué necesita saber antes de que le administren Fentanilo Kalceks
- Cómo se le administra Fentanilo Kalceks
- Posibles efectos adversos
- Conservación de Fentanilo Kalceks
- Contenido del envase e información adicional
1. Qué es Fentanilo Kalceks y para qué se utiliza
Fentanilo Kalceks 50 microgramos/ml, solución inyectable es un líquido que se inyecta. Fentanilo es una sustancia que reduce el dolor y es responsable de la acción de este medicamento. Fentanilo pertenece a un grupo de analgésicos narcóticos potentes, que también se denominan analgésicos opioides.
Este medicamento se le administrará durante la intervención quirúrgica para asegurar que no sienta dolor.
2. Qué necesita saber antes de que le administren Fentanilo Kalceks
No le deben administrarFentaniloKalceks
- si es alérgico a fentanilo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Además, si es hipersensible a otros analgésicos potentes (narcóticos), no debe recibir este medicamento.
- Si sus pulmones no funcionan con normalidad (sin ventilación mecánica).
Advertencias y precauciones
Después de la administración de este medicamento, su respiración se puede volver excesivamente lenta o débil. Es importante que informe inmediatamente a su médico si esto le ocurre. Ya que esto también puede suceder durante el periodo postoperatorio, estará bajo observación durante este periodo.
Antes de que le administren Fentanilo Kalceks, consulte a su médico o enfermerosi:
- tiene insuficiencia hepática, renal o tiroidea;
- tiene enfermedad pulmonar o respiratoria;
- usa alcohol o drogas;
- tiene algún trastorno muscular (miastenia gravis);
- está usando ciertos medicamentos para la depresión (consulte «Otros medicamentos y Fentanilo Kalceks»);
- en pacientes de edad avanzada o debilitados y en niños (ver sección 3);
- usted o alguien de su familia alguna vez ha abusado o ha sido dependiente del alcohol, medicamentos sujetos a prescripción o drogas ilegales (“adicción”);
- es usted fumador;
- alguna vez ha tenido problemas con su estado de ánimo (depresión, ansiedad o un trastorno de la personalidad) o si ha sido tratado por un psiquiatra por otra enfermedad mental.
Informe a su médico si una de estas advertencias se aplica a usted. Puede ser necesaria una estrecha vigilancia médica cuando le administren este medicamento. También puede ser necesario un ajuste de la dosis.
El uso repetido de los analgésicos opioides puede hacer que el medicamento pierda eficacia (usted se acostumbra a él). También puede dar lugar a dependencia y abuso que pueden causar una sobredosis potencialmente mortal. Si usted está preocupado por la posibilidad de adquirir dependencia de Fentanilo Kalceks, es importante que consulte a su médico.
Si se interrumpe el tratamiento, pueden aparecer síntomas de abstinencia. Informe a su médico o enfermero si usted cree que le está pasando esto (ver también la sección 4. Posibles efectos adversos).
Niños
No hay experiencia de uso de este medicamento en niños menores de 2 años. Por lo tanto, no se recomienda administrar este medicamento a niños menores de 2 años.
Otros medicamentos y Fentanilo Kalceks
Informe a su médico o enfermero si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluidos los medicamentos obtenidos sin receta.
Es especialmente importante para los medicamentos enumerados a continuación, ya que puede ser necesario ajustar la dosis de este o de los otros medicamentos, o puede ser necesaria una vigilancia más estrecha.
Informe a su médico si está usando o ha usado recientemente:
- ciertos medicamentos para tratar la depresión:
- inhibidores selectivos de la recaptación de serotonina (ISRS);
- inhibidores de la recaptación de serotonina y norepinefrina (IRSN);
- inhibidores de la monoamino oxidasa (MAO).
Si se usan juntos pueden producir cambios en el estado de ánimo (p. ej., agitación, alucinaciones [percibir cosas que no están allí], coma), temperatura corporal por encima de 38 °C, latidos cardíacos más rápidos, presión arterial inestable y reflejos hiperactivos, rigidez muscular, falta de coordinación y/o síntomas del tracto gastrointestinal (p. ej., náuseas, vómitos, diarrea). Su médico determinará si este medicamento es adecuado para usted.
Si está utilizando un inhibidor de la MAO, siempre que sea posible, su médico suspenderá el tratamiento con estos medicamentos al menos 2 semanas antes de que le administren este medicamento.
- analgésicos potentes durante mucho tiempo;
- algunos analgésicos para el dolor neuropático (gabapentina y pregabalina);
- medicamentos para tratar la psicosis o la enfermedad de Parkinson;
- pastillas para dormir;
- tranquilizantes;
- medicamentos antiepilépticos (por ejemplo, carbamazepina o fenitoína);
- medicamentos para reducir la ansiedad;
- medicamentos para ciertas enfermedades mentales;
- medicamentos para infecciones por hongos (por ejemplo, fluconazol o voriconazol);
- ritonavir (medicamento para el tratamiento de la infección por VIH). Si le dan una dosis única de fentanilo, su médico estará especialmente atento y es posible que le recete una dosis más baja para su uso prolongado.
Fentanilo Kalceks con alcohol
Informe a su médico si está usando o ha usado recientemente alcohol o drogas.
El alcohol puede aumentar ciertos efectos de este medicamento. Este medicamento también influye en el efecto del alcohol. Por estos motivos, no beba alcohol antes de recibir este medicamento ni tampoco el día después de recibir este medicamento.
Embarazo y lactancia
Si está embrazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
No hay suficientes conocimientos sobre si el uso de este medicamento es perjudicial si está embarazada. No se recomienda el uso de fentanilo durante el parto, inclusive durante la cesárea, ya que puede producir problemas respiratorios en el recién nacido.
Lactancia
La sustancia responsable del efecto de este medicamento pasa a la leche materna. Por lo tanto, no se recomienda lactancia materna durante las primeras 24 horas después de la administración de este medicamento. No use leche materna extraída durante las 24 horas posteriores a la administración de este medicamento. Hable con su médico.
Conducción y uso de máquinas
No conduzca un automóvil ni ningún otro vehículo y no utilice máquinas o herramientas durante al menos 24 horas después de haber recibido este medicamento, ya que este medicamento puede afectar su estado de alerta y su capacidad para conducir. Su médico decidirá cuándo puede conducir nuevamente u operar maquinaria peligrosa después de haber recibido este medicamento.
Uso en deportistas
Se informa a los deportistas que este medicamento contiene un componente que puede establecer un resultado analítico del control de dopaje como positivo.
Fentanilo Kalceks contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por ampolla de 2 ml; esto es, esencialmente ”exento de sodio”.
Este medicamento contiene 35,41 mg de sodio (componente principal de la sal para cocinar/de mesa) por ampolla de 10 ml. Esto equivale a 1,78% de la ingesta diaria máxima recomendada de sodio en la dieta para un adulto.
3. Cómo se le administra Fentanilo Kalceks
Este medicamento se administra por inyección en una vena.
Posología
Es importante que reciba la cantidad correcta de este medicamento. La dosis puede variar según la edad, el peso corporal, el estado físico, las enfermedades subyacentes, los medicamentos que toma de forma simultánea y el tipo de anestesia e intervención quirúrgica. Su médico determinará la dosis correcta para usted.
Adultos
Por lo general, se administran 4-12 ml de este medicamento justo antes de la intervención quirúrgica. Si el médico lo considera necesario, se puede administrar otra dosis adicional posteriormente.
Pacientes de edad avanzada y debilitados
La dosis administrada a pacientes de edad avanzada (65 años o más) o a pacientes debilitados justo antes de la intervención quirúrgica es menor que la indicada para otros adultos. Si el médico lo considera necesario, se puede administrar otra dosis adicional posteriormente.
Niños de 2 años de edad y mayores
La dosis administrada a los niños justo antes de la intervención quirúrgica depende del peso del niño. Si el médico lo considera necesario, se puede administrar otra dosis adicional posteriormente.
Los adolescentes de 12 a 17 años reciben la misma dosis que los adultos.
Niños menores de 2 años de edad
No hay experiencia de uso de este medicamento en niños menores de 2 años. Por lo tanto, no se recomienda administrar este medicamento en esta franja de edad.
Pacientes con problemas renales
El médico puede decidir reducir la dosis administrada a pacientes con problemas renales.
Pacientes obesos
La dosis administrada a pacientes obesos justo antes de la intervención quirúrgica puede ser inferior a la indicada para otros adultos. Si el médico lo considera necesario, se puede administrar otra dosis adicional posteriormente.
Si usa más Fentanilo Kalceks del que debiera
Puesto que este medicamento se lo administrará un profesional sanitario es poco probable que le administren demasiado. Sin embargo, informe a su médico o enfermero de inmediato si experimenta respiración superficial o lenta, o si su respiración se detiene temporalmente.
Una sobredosis puede resultar en un trastorno cerebral (conocido como leucoencefalopatía tóxica).
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves. Si tiene alguno de los efectos secundarios enumerados a continuación, su médico debe decidir si su tratamiento debe interrumpirse de inmediato:
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- choque anafiláctico (reacción alérgica severa a ciertas sustancias, en la cual ocurre lo siguiente como resultado de la dilatación repentina y grave de los vasos sanguíneos: caída brusca de la presión arterial, palidez, inquietud, pulso débil y rápido, piel húmeda y pérdida de conciencia);
- síndrome de la serotonina (síndrome con características como inquietud, alucinaciones, coma, palpitaciones cardíacas, presión arterial inestable, temperatura corporal elevada, mayor respuesta a los estímulos, mala coordinación, rigidez, náuseas, vómitos y diarrea).
Otros efectos secundarios. Informe a su médico o enfermero si alguno de los efectos adversos se agrava:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- náuseas, vómitos;
- rigidez muscular.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- movimientos involuntarios, somnolencia, mareos;
- alteraciones visuales;
- latidos cardíacos lentos, latidos cardíacos rápidos, trastornos del ritmo cardíaco;
- reducción de la presión arterial, aumento de la presión arterial, dolor en las venas;
- espasmos en las cuerdas vocales, dificultad para respirar debido a los espasmos de los músculos de las vías respiratorias, respiración superficial o interrumpida;
- inflamación alérgica de la piel;
- confusión después de la intervención quirúrgica, alteraciones del sistema nervioso debido a la anestesia.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- agitación o estado de ánimo eufórico;
- dolor de cabeza;
- inflamación superficial en las venas, fluctuaciones en la presión arterial;
- hiperventilación, hipo;
- dificultad para tragar;
- escalofríos, baja temperatura corporal;
- problemas respiratorios debido a la anestesia, agitación después de la intervención quirúrgica, complicaciones como resultado de dicha intervención.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- hipersensibilidad (que incluye erupción cutánea con picazón y urticaria (ronchas) extensa; la hipersensibilidad a las sustancias del medicamento puede provocar una reacción grave en la que los vasos sanguíneos se ensanchan de repente y hacen que la presión arterial baje y el corazón lata rápida pero débilmente, que se manifiesta como palidez, inquietud y piel húmeda);
- delirio (los síntomas pueden incluir una combinación de agitación, inquietud, desorientación, confusión, miedo, ver o escuchar cosas que realmente no están, trastornos del sueño, pesadillas);
- convulsiones, pérdida de conciencia, contracción muscular repentina (mioclonía);
- paro cardíaco;
- disminución de la fuerza, profundidad o frecuencia de la respiración;
- picor;
- síntomas del síndrome de abstinencia (puede manifestarse por la aparición de los siguientes efectos secundarios: náuseas, vómitos, diarrea, ansiedad, escalofríos, temblor y sudoración).
Se han notificado casos de síndrome serotoninérgico cuando se usó fentanilo junto con ciertos medicamentos para la depresión (ver sección «Otros medicamentos y Fentanilo Kalceks»).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fentanilo Kalceks
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en el embalaje original para protegerlo de la luz. No congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase exterior o en la ampolla después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fentanilo Kalceks
- El principio activo es fentanilo (como citrato de fentanilo).
Cada ml de solución contiene 50 microgramos de fentanilo (como citrato de fentanilo).
Cada ampolla de 2 ml contiene 100 microgramos de fentanilo (como citrato de fentanilo).
Cada ampolla de 10 ml contiene 500 microgramos de fentanilo (como citrato de fentanilo).
- Los demás componentes son cloruro de sodio, hidróxido de sodio (para el ajuste del pH), agua para preparaciones inyectables. Este medicamento no contiene conservantes.
Aspecto de Fentanilo Kalceks y contenido del envase
Solución inyectable transparente e incolora, sin partículas visibles.
10 ampollas de vidrio de 2 ml
10 ampollas de vidrio de 10 ml
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
AS KALCEKS
Krustpils iela 71E, Riga, LV-1057, Letonia
Tel.: +371 67083320
E-mail: [email protected]
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización
EVER Pharma Therapeutics Spain SL
c/ Toledo 170
28005 Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
Los Países Bajos Fentanyl Kalceks 0,05 mg/ml oplossing voor injectie
Austria Fentanyl Kalceks 50 Mikrogramm/ml Injektionslösung
Bulgaria Fentanyl Kalceks 50 ??????????/ml ??????????? ???????
Croacia Fentanil Kalceks
Dinamarca Fentanyl Kalceks
Estonia Fentanyl Kalceks
Finlandia Fentanyl Kalceks
Alemania Fentanyl Kalceks 50 Mikrogramm/ml Injektionslösung
Grecia FENTANYL/KALCEKS
Hungría Fentanyl Kalceks 50 mikrogramm/ml oldatos injekció
Irlanda Fentanyl 50 micrograms/ml solution for injection
Italia Fentanil Kalceks
Noruega Fentanyl Kalceks
Rumanía Fentanil Kalceks 50 micrograme/ml solutie injectabila
Eslovaquia Fentanyl Kalceks 50 mikrogramov/ml injekcný roztok
Eslovenia Fentanil Kalceks 50 mikrogramov/ml raztopina za injiciranje
España Fentanilo Kalceks 50 microgramos/ml solución inyectable EFG
Suecia Fentanyl Kalceks
Reino Unido (Irlanda del Norte) Fentanyl 50 micrograms/ml solution for injection
Fecha de la última revisión de este prospecto: Marzo 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
?----------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Consulte la Ficha técnica o resumen de las características del producto para obtener una descripción completa y otra información.
Indicaciones terapéuticas
Fentanilo Kalceks 50 microgramos/ml es un analgésico y anestésico:
- para su uso como analgésico opioide complementario en la anestesia general o local;
- para su administración con un neuroléptico.
Posología y forma de administración
Fentanilo Kalceks 50 microgramos/ml solamente se debe administrar en un entorno donde sea posible controlar las vías respiratorias y por parte de profesionales que puedan vigilar las vías respiratorias (ver la Ficha Técnica sección 4.4).
La dosis de Fentanilo Kalceks 50 microgramos/ml se debe determinar de forma individual según la edad, peso corporal, estado físico, estado patológico subyacente, uso de otros fármacos y tipo de intervención quirúrgica y de anestesia.
Adultos
En la inducción de la anestesia, se suelen inyectar por vía intravenosa de 200 a 600 microgramos (2,8 a 8,5 microgramos/kg) correspondientes a 4-12 ml. Las dosis superiores a 200 microgramos únicamente se deben administrar con ventilación. Al cabo de 30 a 45 minutos se pueden administrar dosis intravenosas adicionales de 50 a 200 microgramos (de 0,7 a 2,8 microgramos/kg), correspondientes a un volumen de 1‑4 ml, para el mantenimiento de la analgesia.
Población pediátrica
Adolescentes de 12 a 17 años de edad
Seguir la dosis indicada para adultos.
Niños de 2 a 11 años de edad
En general, se recomienda para inducción de la anestesia en niños, una dosis de 1,25-2,5 microgramos/kg o 0,25‑0,5 ml por cada 10 kg de peso. Para el mantenimiento de la analgesia se pueden administrar dosis intravenosas adicionales de 0,25 ml por cada 10 kg cada 30‑45 minutos.
Niños menores de 2 años de edad
No hay experiencia de uso del fentanilo en niños menores de 2 años de edad.
Uso en niños
En niños con respiración espontánea, las técnicas que incluyen analgesia sólo se deben utilizar como parte de una técnica anestésica o administrarse como parte de una técnica de sedación/analgesia por personal experimentado y en un entorno que permita tratar una rigidez muscular repentina (que requiera intubación), o una apnea (que requiera ventilación) (ver la Ficha Técnica sección 4.4).
Uso en ancianos
Al igual que con otros opioides, la dosis inicial para ancianos (> 65 años) y pacientes debilitados se debe reducir. El efecto de la dosis inicial se debe tener en cuenta al determinar las dosis adicionales.
Uso en pacientes con insuficiencia renal
En pacientes con insuficiencia renal se debe considerar una reducción en la dosis de Fentanilo Kalceks 50 microgramos/ml y se debe observar cuidadosamente a estos pacientes para detectar signos de toxicidad del fentanilo (ver la Ficha Técnica sección 5.2).
Uso en pacientes obesos
En pacientes obesos hay riesgo de sobredosis si la dosis se calcula a partir del peso corporal. La dosis para pacientes obesos (IMC > 30 kg/m2) se debe calcular a partir de la masa magra corporal estimada en lugar del peso corporal solo. Para cualquier ajuste posológico posterior se debe proceder con cautela en función del efecto (ver la Ficha Técnica sección 5.2).
Forma de administración
Administrar por vía intravenosa lenta (durante 1 a 2 minutos).
Contraindicaciones
- Hipersensibilidad al principio activo o a alguno de los excipientes incluidos en la sección 6, o a otros opioides.
- Insuficiencia respiratoria sin ventilación mecánica, debido al efecto depresor respiratorio específico de los fármacos morfinomiméticos.
Advertencias y precauciones especiales de empleo
- El fentanilo solamente se debe administrar en un entorno donde sea posible controlar las vías respiratorias y por parte de profesionales que puedan vigilar las vías respiratorias.
- Tal como ocurre con todos los opioides potentes, el fentanilo puede producir depresión respiratoria que depende de la dosis. Tras la administración de fentanilo en dosis superiores a 200microgramos de fentanilo (4ml) se producirá una depresión respiratoria significativa. Este efecto farmacológico puede ser revertido por la naloxona, un antagonista específico de los opioides. En ocasiones puede ser necesario repetir la dosis del antagonista de los opioides, ya que la depresión respiratoria puede durar más tiempo que la acción de estos antagonistas. La analgesia profunda se acompaña de una depresión respiratoria manifiesta que puede persistir o repetirse en el periodo postoperatorio. Por lo tanto, es importante que los pacientes permanezcan bajo la debida vigilancia. El equipo de reanimación y los antagonistas de los opioides deben estar disponibles de inmediato. La hiperventilación durante la anestesia puede alterar la respuesta del paciente al CO2, afectando así, la respiración en el periodo posoperatorio.
- Puede producirse rigidez muscular, a raíz de la cual puede aparecer una depresión respiratoria. La incidencia puede reducirse con una inyección intravenosa lenta (que suele ser suficiente para dosis bajas). La reacción se puede tratar con ventilación mecánica, premedicación con una benzodiazepina y, si es necesario, administración de relajantes musculares.
- Cuando se administra fentanilo se debe tener en cuenta que puede ocurrir una reacción anafiláctica.
- Pueden aparecer movimientos mioclónicos no epilépticos.
- Puede producirse bradicardia y fallo cardíaco si el paciente recibe una cantidad insuficiente de anticolinérgico o al combinar este medicamento con relajantes musculares no vagolíticos. La bradicardia puede tratarse administrando atropina.
- Los opioides pueden causar hipotensión, especialmente en pacientes con hipovolemia. Deben adoptarse las medidas adecuadas para mantener una presión arterial estable.
- Debe evitarse la inyección rápida (en embolada) de opioide. En pacientes con alteración de la distensibilidad intracerebral, la reducción transitoria de la presión arterial media se ha acompañado a veces de una reducción durante un corto periodo de tiempo del riego sanguíneo cerebral.
- Los pacientes que reciben un tratamiento crónico con opioides o aquellos con adicción a los mismos podrían requerir dosis mayores.
- Se recomienda reducir la dosis en pacientes de edad avanzada o debilitados. En casos de hipotiroidismo no controlado, enfermedad pulmonar, insuficiencia respiratoria o alcoholismo se debe ajustar cuidadosamente la dosis de los opioides, así como la de los pacientes con insuficiencia hepática, debido a una posible alteración del metabolismo. Se debe vigilar cuidadosamente a los pacientes con insuficiencia renal para detectar cualquier síntoma de toxicidad del fentanilo. El volumen de distribución del fentanilo puede variar como resultado de la diálisis, lo que puede afectar a las concentraciones plasmáticas. Se debe someter a estos pacientes a observación postoperatoria durante un periodo más prolongado.
- Si este medicamento se administra junto con neurolépticos, el profesional sanitario debe estar familiarizado con las propiedades específicas de cada fármaco, en particular con las diferencias en la duración de la acción. El riesgo de hipotensión es mayor cuando se administra esta combinación. Los neurolépticos pueden dar lugar a síntomas extrapiramidales que se pueden contrarrestar con antiparkinsonianos, aunque la combinación con estos últimos puede aumentar el riesgo de discinesia tardía.
- Al igual que con otros opioides, debido a los efectos anticolinérgicos, la administración de fentanilo puede provocar un incremento de la presión en el conducto biliar y ocasionalmente, se pueden observar espasmos del esfínter de Oddi.
- En los pacientes con miastenia grave, debe considerarse cuidadosamente el uso de ciertos anticolinérgicos y bloqueantes neuromusculares, antes y durante la administración de una pauta posológica de anestesia general que incluya la administración de fentanilo intravenoso.
- Es necesario actuar con precaución cuando se administra este medicamento simultáneamente con fármacos que afectan los sistemas neurotransmisores serotoninérgicos.
Con el uso concomitante de fármacos serotoninérgicos, como ISRS e inhibidores de la recaptación de serotonina y norepinefrina (IRSN), y con fármacos que afectan el metabolismo de la serotonina (incluidos los inhibidores de la monoaminoxidasa [MAO]), se puede presentar un síndrome serotoninérgico potencialmente mortal. Esto puede ocurrir utilizando las dosis recomendadas.
El síndrome serotoninérgico puede incluir cambios en el estado psíquico (por ejemplo: agitación, alucinaciones o coma), inestabilidad autonómica (por ejemplo: taquicardia, presión arterial lábil o hipertermia), alteraciones neuromusculares (por ejemplo: hiperreflexia, falta de coordinación o rigidez) y/o síntomas gastrointestinales (por ejemplo: náuseas, vómitos o diarrea).
Si se sospecha que existe un síndrome serotoninérgico se debe considerar la rápida interrupción del tratamiento con este medicamento.
Tolerancia y trastorno por consumo de opioides (abuso y dependencia)
Puede aparecer tolerancia, dependencia física y psicológica tras la administración repetida de opioides.
El uso repetido de opioides puede conducir a un trastorno por consumo de opioides (TCO). El abuso o mal uso intencionado de los opioides puede causar sobredosis y/o muerte. El riesgo de desarrollar un TCO aumenta en los pacientes con antecedentes personales o familiares (padres o hermanos) de trastornos por consumo de sustancias (incluido el trastorno por consumo de alcohol), en los fumadores activos o en los pacientes con antecedentes personales de otros trastornos de salud mental (p. ej., depresión mayor, ansiedad y trastornos de la personalidad).
Síndrome de abstinencia
La administración repetida a intervalos cortos durante períodos prolongados puede dar lugar al desarrollo de un síndrome de abstinencia tras la interrupción del tratamiento, que se puede manifestar por la aparición de los siguientes efectos adversos: náuseas, vómitos, diarrea, ansiedad, escalofríos, temblores y sudoración.
Población pediátrica
En niños con respiración espontánea, las técnicas que incluyen analgesia sólo se deben utilizar como parte de una técnica anestésica o administrarse como parte de una técnica de sedación/analgesia por personal experimentado y en un entorno que permita tratar una rigidez muscular repentina (que requiera intubación), o una apnea (que requiera ventilación).
Uso en deportistas
Este medicamento contiene fentanilo que puede producir un resultado positivo en las pruebas de control de dopaje.
Excipientes
Este medicamento contiene:
7,08 mg de sodio menos de 23 mg de sodio (1 mmol) por cada ampolla de 2 ml, esto es esencialmente «exento de sodio».
35,41 mg de sodio por cada ampolla de 10 ml, equivalente a 1,78% de la ingesta diaria máxima recomendada por la OMS de 2 g de sodio para un adulto.
Interacción con otros medicamentos y otras formas de interacción
Efecto de otros fármacos sobre el fentanilo
Inhibidores de la MAO y otros fármacos serotoninérgicos
La coadministración de fentanilo con inhibidores de la MAO, puede producir estimulación paroxística del SNC e hipertensión. La administración simultánea debe evitarse e interrumpir en lo posible el tratamiento con los inhibidores de la MAO durante al menos 2 semanas antes de iniciar el tratamiento con este medicamento.
El uso concomitante de fentanilo con fármacos serotoninérgicos, como los ISRS y los IRSN, y con inhibidores de la monoaminoxidasa (IMAO) puede incrementar el riesgo de desarrollar un síndrome serotoninérgico potencialmente mortal.
Si el uso simultáneo de este medicamento con los ISRS, IRSN o los IMAO es inevitable, debe vigilarse la aparición en el paciente de síntomas del síndrome serotoninérgico durante la coadministración.
El uso de barbitúricos, benzodiacepinas, neurolépticos, gases halogenados, los gabapentinoides (gabapentina y pregabalina) y otros depresores no selectivos del SNC (incluido el alcohol) puede potenciar la depresión respiratoria causada por los opioides. Si los pacientes han recibido depresores del SNC, la dosis necesaria de fentanilo será inferior a la habitual.
El fentanilo, un principio activo de aclaramiento elevado, es metabolizado de forma rápida y extensa por el CYP3A4. El itraconazol (un potente inhibidor del CYP3A4) administrado a 200 mg/día por vía oral durante cuatro días no ejerció un efecto significativo sobre la farmacocinética del fentanilo intravenoso. La administración oral del ritonavir (uno de los inhibidores más potentes del CYP3A4) redujo el aclaramiento del fentanilo administrado por vía intravenosa en dos tercios. No obstante, las concentraciones plasmáticas máximas no se vieron afectadas tras la administración de una dosis intravenosa única de fentanilo.
La coadministración de fluconazol o voriconazol y fentanilo puede aumentar la exposición al fentanilo del 25% al 40%, aproximadamente. Durante el uso conjunto de fluconazol o voriconazol y fentanilo se debe vigilar estrechamente a los pacientes, ajustando la dosis de fentanilo según sea necesario.
Cuando se administra fentanilo en una dosis única oral, es necesaria una atención especial y observación del paciente al usar inhibidores potentes del CYP3A4, como el ritonavir, de forma simultánea. Con una administración continua, puede ser necesario reducir la dosis de fentanilo para evitar su acumulación, lo cual, en ciertos casos, aumenta el riesgo de depresión respiratoria prolongada o retardada.
Inductores del citocromo P450 3A4 (CYP3A4)
Una inyección de fentanilo junto con la administración de inductores potentes del CYP3A4 (por ejemplo, la carbamazepina o la fenitoína), puede disminuir las concentraciones plasmáticas del fentanilo, reduciendo, por tanto, su eficacia. Se debe vigilar de cerca al paciente para detectar indicios de reducción de los efectos analgésicos si el fentanilo se utiliza junto con un fuerte inductor del CYP3A4. También debe considerarse el aumento de la dosis de fentanilo, si es necesario.
Efectos del fentanilo sobre otros fármacos
El uso simultáneo de otros fármacos con efecto depresor del sistema nervioso central, como opioides, sedantes, hipnóticos, anestésicos generales, fenotiazinas, tranquilizantes, relajantes musculares, antihistamínicos sedantes y las bebidas alcohólicas podrían producir efectos depresores aditivos y podrían darse casos de hipoventilación, hipotensión y sedación profunda o coma. Por lo tanto, el uso del fentanilo con cualquiera de los medicamentos anteriormente indicados requiere una atención especial y una observación del paciente.
Con el uso concomitante del fentanilo, las concentraciones plasmáticas de etomidato aumentaron considerablemente (en un factor de 2‑3). Durante el uso simultáneo, el aclaramiento total del plasma y el volumen de distribución del etomidato disminuyen en un factor de 2 a 3 sin ningún cambio en la semivida.
La administración conjunta de fentanilo y midazolam intravenoso da lugar a un aumento de la semivida plasmática terminal y a una disminución de la eliminación plasmática del midazolam. La exposición al midazolam aumenta aproximadamente en un 50%. El mecanismo de interacción es la inhibición competitiva del CYP3A4 (ver la Ficha Técnica sección 5.2). Cuando el midazolam se administra conjuntamente con el fentanilo, puede ser necesario reducir la dosis de midazolam.
Incompatibilidades
Este medicamento no debe mezclarse con otros medicamentos.
Precauciones especiales de eliminación y otras manipulaciones
Para un solo uso. Si se usa solamente en parte, desechar la solución restante.
Proteger los dedos al abrir una ampolla.
Después de la primera apertura: el medicamento debe usarse inmediatamente.
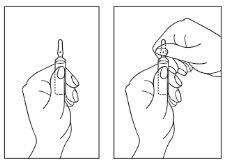
Instrucciones para la apertura de la ampolla:
- Mantenga la ampolla con el punto de color hacia arriba. Si queda algo de solución en la parte superior de la ampolla, golpee suavemente con el dedo para que toda la solución baje a la parte inferior.
- Use ambas manos para abrirla y mientras sostiene la parte inferior de la ampolla con una mano, use la otra para romper la parte superior de la ampolla en dirección opuesta al punto de color (ver las imágenes a continuación).
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FENTANILO KALCEKS 50 MICROGRAMOS/ML SOLUCION INYECTABLE EFGForma farmacéutica: INYECTABLE, 0,0785 mg/mlPrincipio activo: fentaniloFabricante: Kern Pharma S.L.Requiere recetaForma farmacéutica: INYECTABLE, 50 MICROGRAMOSPrincipio activo: fentaniloFabricante: Laboratorios Basi Industria Farmaceutica S.A.Requiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 30 MICROGRAMOSPrincipio activo: sufentanilFabricante: Laboratoire AguettantRequiere receta
Médicos online para FENTANILO KALCEKS 50 MICROGRAMOS/ML SOLUCION INYECTABLE EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FENTANILO KALCEKS 50 MICROGRAMOS/ML SOLUCION INYECTABLE EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















