
DZUVEO 30 MICROGRAMOS COMPRIMIDO SUBLINGUAL


Cómo usar DZUVEO 30 MICROGRAMOS COMPRIMIDO SUBLINGUAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Dzuveo 30microgramos comprimido sublingual
sufentanilo
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Dzuveo y para qué se utiliza
- Qué necesita saber antes de empezar a usar Dzuveo
- Cómo usar Dzuveo
- Posibles efectos adversos
- Conservación de Dzuveo
- Contenido del envase e información adicional
1. Qué es Dzuveo y para qué se utiliza
El principio activo de Dzuveo es el sufentanilo, que pertenece a un grupo de medicamentos potentes para el alivio del dolor denominados opioides.
El sufentanilo se utiliza para tratar el dolor repentino moderado a intenso en adultos en un ámbito controlado médicamente, como es un hospital.
2. Qué necesita saber antes de empezar a usar Dzuveo
No use Dzuveo
- Si es alérgico al sufentanilo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene problemas pulmonares o respiratorios graves.
Advertencias y precauciones
Consulte a su médico o enfermero antes de empezar a usar Dzuveo. Informe a su médico o enfermero antes del tratamiento si:
- Presenta algún trastorno que afecte a su respiración (como asma, sonido silbante que se produce al respirar [sibilancias] o dificultad para respirar). Como Dzuveo puede afectar a su respiración, durante el tratamiento su médico o enfermero comprobarán su respiración.
- Presenta un traumatismo craneoencefálico o un tumor cerebral.
- Tiene problemas de corazón y de circulación, especialmente frecuencia cardíaca lenta, latidos cardíacos irregulares, volumen sanguíneo bajo o presión arterial baja.
- Tiene problemas de moderados a graves en el hígado o problemas graves en el riñón, ya que estos órganos afectan a la forma en que su organismo descompone y elimina el medicamento; tiene movimientos intestinales anormalmente lentos.
- Padece una enfermedad de la vesícula biliar o del páncreas.
- Usted o cualquier miembro de su familia alguna vez ha abusado o tenido dependencia de alcohol, de medicamentos con receta o de drogas ilegales («adicción»).
- Es fumador.
- Ha tenido alguna vez problemas relacionados con el estado de ánimo (depresión, ansiedad o un trastorno de la personalidad) o ha sido tratado por un psiquiatra por otras enfermedades mentales.
Este medicamento contiene sufentanilo, que es un opioide. El uso repetido de analgésicos opioides puede hacer que el fármaco sea menos eficaz (el organismo se acostumbra a ellos). También puede producir dependencia y abuso, lo que puede dar lugar a una sobredosis potencialmente mortal. Es importante que consulte a su médico si le preocupa llegar a desarrollar dependencia de Dzuveo.
Consulte a su médico DURANTE el uso de Dzuveo si:
- Siente dolor o mayor sensibilidad al dolor (hiperalgesia) que no responde a una dosis más alta del medicamento tal como se lo recetó el médico.
Qué necesita saber antes de empezar a usar Dzuveo
Trastornos respiratorios relacionados con el sueño
- Dzuveo puede causar trastornos respiratorios con el sueño tales como la apnea del sueño (pausas respiratorias durante el sueño) e hipoxemia relacionada con el sueño (nivel de oxígeno en la sangre bajo). Estos síntomas pueden incluir pausas respiratorias durante el sueño, despertar nocturno debido a dificultad para respirar, dificultad para mantener el sueño o somnolencia excesiva durante el día. Si usted u otra persona observan estos síntomas, contacte a su médico. Su medico pudiera considerar una reducción de la dosis.
Niños y adolescentes
Dzuveo no se debe utilizar en niños y adolescentes menores de 18 años.
Otros medicamentos y Dzuveo
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. En particular, informe a su médico si está tomando alguno de los siguientes:
- Ketoconazol, utilizado para el tratamiento de las infecciones por hongos, este medicamento puede afectar a la forma en que su organismo descompone el sufentanilo.
- Cualquier medicamento que pudiera adormecerle (tener un efecto sedante), como pastillas para dormir, medicamentos para tratar la ansiedad (p. ej., benzodiacepinas), tranquilizantes u otros medicamentos opioides, ya que podrían aumentar el riesgo de problemas respiratorios graves, coma y muerte.
- Medicamentos para el tratamiento de la depresión, conocidos como inhibidores de la monoaminooxidasa (IMAO). Estos medicamentos no deben tomarse en las 2 semanas previas, o de forma concomitante con la administración de Dzuveo.
- Medicamentos para el tratamiento de la epilepsia, el dolor de origen neurológico o la ansiedad (gabapentina y pregabalina), ya que aumentan el riesgo de sobredosis de opioides ydepresión respiratoria y pueden poner en peligro la vida del paciente.
- Medicamentos para el tratamiento de la depresión conocidos como inhibidores selectivos de la recaptación de serotonina (ISRS) e inhibidores de la recaptación de serotonina y noradrenalina (IRSN). No se recomienda utilizar estos medicamentos de forma concomitante con Dzuveo.
- Otros medicamentos que también se tomen por vía sublingual (medicamentos que se colocan debajo de la lengua, donde se disuelven) o medicamentos que tengan efecto en su boca (como la nistatina, un líquido o pastillas que se mantienen en la boca para tratar las infecciones por hongos), ya que no se ha estudiado el efecto sobre Dzuveo.
- Opioides recetados habitualmente (p. ej., morfina, codeína, fentanilo, hidromorfona, oxicodona).
- Medicamentos utilizados para tratar la hipertensión o la angina (dolor torácico), conocidos como antagonistas del calcio o betabloqueantes, como diltiazem y nifedipino.
Uso de Dzuveo con alcohol
No beba alcohol mientras esté usando Dzuveo. Puede aumentar el riesgo de presentar problemas respiratorios graves.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de tomar este medicamento.
Dzuveo no debe utilizarse durante el embarazo ni en mujeres en edad fértil que no estén utilizando métodos anticonceptivos eficaces.
Dzuveo pasa a la leche materna y puede ocasionar efectos adversos en el lactante. No se recomienda la lactancia si está tomando Dzuveo.
Conducción y uso de máquinas
Dzuveo afecta a su capacidad para conducir o utilizar máquinas, ya que puede causar sueño, mareo o alteraciones visuales. No debe conducir ni utilizar máquinas si experimenta alguno de estos síntomas durante o después del tratamiento con sufentanilo. Solo debe conducir y utilizar máquinas si ha transcurrido un tiempo suficiente después de la última administración de Dzuveo.
Dzuveo contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo usar Dzuveo
Este medicamento debe ser administrado por un médico o un enfermero utilizando el dispositivo de administración unidosis. No debe administrarse usted mismo este medicamento.
Dzuveo solo debe utilizarse en un ámbito controlado médicamente, como es un hospital. Solo pueden recetarlo médicos con experiencia en el uso de analgésicos potentes como el sufentanilo y que conozcan los efectos que podría tener en usted, particularmente en su respiración (ver «Advertencias y precauciones» más arriba).
La dosis recomendada es de un comprimido sublingual de 30 microgramos como máximo administrado cada hora. Un profesional sanitario le administrará el comprimido sublingual utilizando el aplicador unidosis desechable. El aplicador ayudará al profesional sanitario a colocar un comprimido debajo de su lengua. Los comprimidos se disuelven debajo de la lengua y no se deben masticar ni tragar porque no serán eficaces para aliviar el dolor salvo que puedan disolverse debajo de la lengua. No debe comer ni beber nada y debe hablar lo menos posible en los 10 minutos siguientes a la administración de cada dosis.
Después de recibir una dosis, no se le administrará otra dosis hasta por lo menos una hora después. La dosis máxima diaria es de 720 microgramos al día (24 comprimidos al día).
Dzuveo no debe utilizarse durante más de 48 horas.
Después de su tratamiento, el personal médico eliminará el aplicador según corresponda.
Si usa más Dzuveo del que debe
Los síntomas de sobredosis incluyen problemas respiratorios graves, como respiración lenta y superficial, pérdida del conocimiento, presión arterial extremadamente baja, colapso y rigidez muscular. Si estos síntomas empiezan a aparecer, informe inmediatamente a un médico o enfermero.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Los efectos adversos más graves son problemas respiratorios graves, como respiración lenta y superficial, que incluso pueden hacer que deje de respirar.
Si presenta alguno de los efectos adversos mencionados anteriormente, informe inmediatamente a su médico o enfermero.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
Náuseas, vómitos o ganas de vomitar y sensación de calor en general.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Incapacidad o dificultad para dormir, ansiedad o confusión, mareo.
- Dolor de cabeza, somnolencia, sensación de sueño.
- Aumento de la frecuencia cardíaca, presión arterial alta, presión arterial baja.
- Niveles bajos de oxígeno en la sangre, sensación de dolor en la parte inferior de la garganta, respiración lenta y superficial.
- Sequedad de boca, flatulencia (gases), estreñimiento, indigestión o reflujo.
- Reacciones alérgicas, picor de la piel.
- Contracciones y espasmos musculares.
- Incapacidad para orinar.
- Este medicamento también puede causar cambios en los niveles de glóbulos rojos, glóbulos blancos, calcio, albúmina, potasio y sodio en la sangre, que solo pueden detectarse mediante un análisis de sangre. Si le van a hacer un análisis de sangre, asegúrese de que su médico sepa que está tomando este medicamento.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Inflamación de los pulmones, enrojecimiento e inflamación de los ojos, inflamación de la garganta.
- Acumulaciones de grasa debajo de la piel.
- Incapacidad para controlar el azúcar en sangre (diabetes), aumento del colesterol.
- Agitación, falta de interés o emoción, falta de energía, desorientación, euforia, alucinaciones o ver cosas que no existen, nerviosismo.
- Problemas de coordinación de los movimientos musculares, contracciones musculares, temblores o agitación excesiva, exageración de las respuestas reflejas, sensación de quemazón, sensación de desvanecimiento, sensación anormal de la piel (hormigueo), entumecimiento en general, cansancio, olvidos, migraña, cefalea tensional.
- Trastornos de la visión, dolor ocular.
- Disminución de la frecuencia cardíaca, latidos cardíacos irregulares, angina u otras molestias torácicas.
- Presión arterial alta o presión arterial baja al ponerse de pie, rubefacción de la piel.
- Respiración lenta o difícil (incluso al dormir), hemorragia nasal, hipo.
- Dolor torácico y dificultad para respirar causados por un coágulo de sangre en el pulmón, líquido en los pulmones, sibilancias.
- Diarrea, eructos, inflamación del revestimiento del estómago o gastritis, gases, reflujo ácido, arcadas, dolor de estómago o molestias de estómago.
- Aparición de ampollas, sudoración excesiva, erupción cutánea, sequedad de la piel, entumecimiento de la boca o la cara.
- Dolor de espalda, tórax u otras partes del cuerpo, dolor en las extremidades.
- Dificultad para orinar, olor intenso de la orina, dolor al orinar, insuficiencia renal.
- Hinchazón, sensaciones incómodas en el pecho, escalofríos y debilidad (falta de energía).
Este medicamento también puede causar cambios en los niveles de plaquetas (que ayudan a la coagulación de la sangre), magnesio, proteínas, azúcar, grasas, fosfatos y plasma en la sangre, que solo pueden identificarse mediante un análisis de sangre. Si le van a hacer un análisis de sangre, asegúrese de que su médico sepa que está tomando este medicamento.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- Reacciones alérgicas graves (shock anafiláctico), convulsiones, coma, tamaño pequeño de las pupilas, enrojecimiento de la piel.
- Síndrome de abstinencia, que puede incluir síntomas como agitación, ansiedad, dolores musculares, insomnio, sudoración y bostezos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Dzuveo
Mantener este medicamento fuera de la vista y del alcance de los niños. Su médico o enfermero se asegurarán de que:
- este medicamento no se utilice después de la fecha de caducidad que aparece en la etiqueta y en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
- Conservar en el embalaje original para protegerlo de la luz y el oxígeno.
- No utilice este medicamento si observa signos de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Su profesional sanitario se deshará de los envases y de los medicamentos que no necesite de acuerdo con las normas del hospital. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Dzuveo
- El principio activo es el sufentanilo. Cada comprimido sublingual contiene 30 microgramos de sufentanilo (como citrato).
- Los demás componentes son manitol (E421), fosfato dicálcico, hipromelosa, croscarmelosa sódica, carmín de índigo (E132), ácido esteárico y estearato de magnesio.
Aspecto del producto y contenido del envase
Dzuveo es un comprimido sublingual plano de color azul, con bordes redondeados. Mide 3 mm de diámetro y se suministra en un aplicador unidosis (etiquetado con [sublingual tablet]). El aplicador, con el comprimido en su interior, se acondiciona en una bolsita.
Cada bolsita contiene un aplicador y un comprimido de sufentanilo 30 microgramos. Cada envase contiene 5 o 10 bolsitas.
Puede que solo estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y Responsable de la fabricación
Laboratoire Aguettant
1, rue Alexander Fleming
69007 Lyon
Francia.
Fecha de la última revisión de este prospecto:
<------------------------------------------------------------------------------------------------------------------------>
Esta información está destinada únicamente a profesionales sanitarios:
Instrucciones de uso del aplicador unidosis (AUD)
Producto de un solo uso / No reutilizar
No usar si se ha roto el precinto de la bolsita
No usar si el aplicador unidosis (AUD) está dañado
Se debe indicar al paciente que no mastique ni trague el comprimido.
Se debe indicar al paciente que no coma ni beba nada y que hable lo menos posible en los 10 minutos siguientes a la administración del comprimido.
- Cuando esté listo para administrar el medicamento, abra la bolsita por la línea de corte en la parte superior. La bolsita contiene un AUD de plástico transparente con un solo comprimido de color azul alojado en la punta y un saquito con absorbente de oxígeno. El saquito con el absorbente de oxígeno debe desecharse.
A continuación se muestra el contenido de la bolsita:
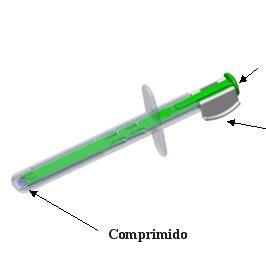
Pulsador
Sistema de bloqueo
- Retire el sistema de bloqueo blanco del pulsador verde presionando sobre ambos lados a la vez para desprenderlo del pulsador. Deseche el sistema de bloqueo.
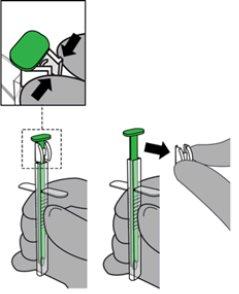
- Indique al paciente que, si es posible, se toque el paladar con la lengua.
- Apoye con suavidad el AUD en los dientes o los labios del paciente.
- Coloque la punta del AUD debajo de la lengua y orientada hacia el suelo de la boca del paciente. NOTA: Evite el contacto directo de la mucosa con la punta del AUD.
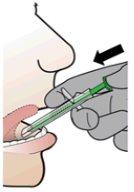
- Presione sobre el pulsador verde para liberar el comprimido en el espacio sublingual del paciente y confirme la correcta colocación del comprimido.
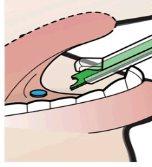
El aplicador unidosis (AMD) debe desecharse de conformidad con las políticas del centro y los requisitos locales.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DZUVEO 30 MICROGRAMOS COMPRIMIDO SUBLINGUALForma farmacéutica: INYECTABLE, 5 microgramos/mlPrincipio activo: sufentanilFabricante: Medochemie LimitedRequiere recetaForma farmacéutica: INYECTABLE, 50 microgramos/mlPrincipio activo: sufentanilFabricante: Medochemie LimitedRequiere recetaForma farmacéutica: INYECTABLE, 5 MICROGRAMOS/MLPrincipio activo: sufentanilFabricante: Altan Pharmaceuticals SaRequiere receta
Médicos online para DZUVEO 30 MICROGRAMOS COMPRIMIDO SUBLINGUAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DZUVEO 30 MICROGRAMOS COMPRIMIDO SUBLINGUAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















