
DEXMEDETOMIDINA KALCEKS 100 MICROGRAMOS/ML CONCENTRADO PARA SOLUCION PARA PERFUSION EFG

Cómo usar DEXMEDETOMIDINA KALCEKS 100 MICROGRAMOS/ML CONCENTRADO PARA SOLUCION PARA PERFUSION EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
DexmedetomidinaKalceks100microgramos/ml concentrado parasolución para perfusión EFG
Dexmedetomidina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Dexmedetomidina Kalceks y para qué se utiliza
- Qué necesita saber antes de que le administren Dexmedetomidina Kalceks
- Cómo usar Dexmedetomidina Kalceks
- Posibles efectos adversos
- Conservación de Dexmedetomidina Kalceks
- Contenido del envase e información adicional
1. Qué es Dexmedetomidina Kalceks y para qué se utiliza
Dexmedetomidina Kalceks contiene una sustancia activa llamada dexmedetomidina, que pertenece a un grupo de medicamentos llamados sedantes. Se utiliza para proporcionar sedación (un estado de calma, somnolencia o sueño) en pacientes adultos en las unidades de cuidados intensivos de los hospitales o sedación consciente durante diferentes procedimientos de diagnóstico o quirúrgicos.
2. Qué necesita saber antes de que le administren Dexmedetomidina Kalceks
No deben administrarle Dexmedetomidina Kalceks:
- si es alérgico a la dexmedetomidina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene algunos trastornos del ritmo cardíaco (bloqueo cardíaco de grado 2 ó 3).
- si tiene una presión sanguínea muy baja que no responda a tratamiento.
- si recientemente ha tenido ictus u otros episodios graves que afectan el aporte de sangre al cerebro.
Advertencias y precauciones
Antes de usar este medicamento, informe a su médico o enfermero si se encuentra en alguna de las siguientes situaciones, ya que Dexmedetomidina Kalceks se debe utilizar con precaución:
- si tiene un ritmo cardíaco anormalmente lento (ya sea debido a enfermedad o a un nivel elevado de su condición física) ya que puede aumentar el riesgo de parada cardíaca
- si tiene la presión arterial baja
- si tiene bajo volumen de sangre, por ejemplo después de una hemorragia
- si tiene ciertas enfermedades del corazón
- si tiene una edad avanzada
- si tiene un trastorno neurológico (por ejemplo, lesiones de la cabeza o de la médula espinal o accidente cerebrovascular)
- si tiene problemas graves del hígado
- si alguna vez ha desarrollado una fiebre grave después de algunos medicamentos, especialmente los anestésicos
Este medicamento puede causar una gran cantidad de orina y sed excesiva, contacte a un médico si ocurren estos efectos adversos. Consulte la sección 4 para más información.
Uso de Dexmedetomidina Kalceks con otros medicamentos
Informe a su médico o enfermero si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Los siguientes medicamentos pueden aumentar el efecto de Dexmedetomidina Kalceks:
- medicamentos que ayudan a dormir o causan sedación (p.ej., midazolam, propofol)
- medicamentos para el dolor fuerte (p.ej., opioides como la morfina, codeína)
- medicamentos anestésicos (p.ej., sevoflurano, isoflurano)
Si usted está usando medicamentos que disminuyen la presión de su sangre y su frecuencia cardíaca, la administración conjunta con Dexmedetomidina Kalceks puede incrementar este efecto. Dexmedetomidina Kalceks no se debe usar con medicamentos que pueden causar parálisis temporal.
Embarazo y lactancia
Dexmedetomidina Kalceks no debe utilizarse durante el embarazo o la lactancia, a menos que sea claramente necesario. Consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Dexmedetomidina Kalceks tiene un impacto importante en la habilidad para conducir y usar máquinas. Una vez se le haya administrado Dexmedetomidina Kalceks no debe conducir, operar máquinas o trabajar en situaciones peligrosas hasta que los efectos hayan pasado completamente. Consulte con su médico cuándo podrá retomar estas actividades y este tipo de trabajo.
Dexmedetomidina Kalcekscontiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por cada ml; esto es, esencialmente “exento de sodio”.
3. Cómo usar Dexmedetomidina Kalceks
Cuidados Intensivos hospitalarios
Dexmedetomidina Kalceks se le administra por un médico o un enfermero en la unidad de cuidados intensivos de un hospital.
Sedación de procedimiento / sedación consciente
Dexmedetomidina Kalceks lo administra un médico o un enfermero antes y/o durante procedimientos de diagnóstico o quirúrgicos que requieren sedación, es decir, para la sedación de procedimientos / sedación consciente.
Su médico decidirá la dosis adecuada para usted. La cantidad de Dexmedetomidina Kalceks depende de su edad, corpulencia, estado general de salud, el nivel de sedación necesario y cómo responde al medicamento. Su médico puede cambiar su dosis si es necesario y controlará su corazón y su presión arterial durante el tratamiento.
Dexmedetomidina Kalceks se diluye y se le administra como una perfusión (goteo) en sus venas.
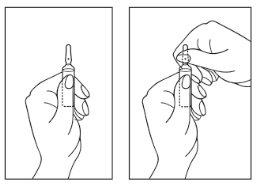
Instrucciones de apertura de ampolla:
- Gire la ampolla con el punto de color hacia arriba. Si hay alguna gota de la solución en la parte superior de la ampolla, golpee suavemente con el dedo para llevar toda la solución a la parte inferior de la ampolla.
- Use ambas manos para abrir. Mientras sostiene la parte inferior de la ampolla con una mano, use la otra mano para partir la parte superior de la ampolla en la dirección opuesta al punto coloreado (vea las imágenes a continuación).
Después de la sedación/despertar
- Su médico le mantendrá bajo supervisión durante algunas horas tras la sedación, para asegurarse que usted se encuentra bien.
- No debe irse a casa si no va acompañado.
- Los medicamentos que ayudan a dormir, causan sedación a su vez aquéllos destinados a calmar el dolor intenso puede que no estén recomendados durante un período de tiempo después del tratamiento con Dexmedetomidina Kalceks. Consulte con su médico sobre el uso de este tipo de medicamentos y sobre el uso de alcohol.
Si le han administrado más Dexmedetomidina Kalceks del que debe
Si se han dado demasiado Dexmedetomidina Kalceks, su presión arterial puede subir o bajar, los latidos de su corazón pueden ser más lentos, puede que respire más lentamente y se puede sentir más somnoliento. Su médico sabrá cómo tratarlo teniendo en cuenta su estado.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes (afectan a más de 1 de cada 10 usuarios)
- frecuencia cardíaca lenta
- presión arterial baja o alta
- cambio en el patrón respiratorio o detención de la respiración
Frecuentes (afectan entre 1 y 10 de cada 100 usuarios)
- dolor de pecho o ataque al corazón
- frecuencia cardíaca rápida
- niveles bajos o altos de azúcar en la sangre
- náuseas, vómitos o sequedad en la boca
- inquietud
- temperatura alta
- síntomas después de dejar el medicamento
Poco frecuentes (afectan entre 1 y 10 de cada 1.000 usuarios)
- función del corazón disminuida, parada cardíaca
- hinchazón del estómago
- sed
- una condición en la que hay demasiado ácido en el cuerpo
- nivel bajo de albúmina en la sangre
- dificultad para respirar
- alucinaciones
- el medicamento no es lo suficientemente eficaz
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- gran cantidad de orina y sed excesiva – pueden ser síntomas de un trastorno hormonal llamado diabetes insípida. Contacte con un médico si esto ocurre.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Dexmedetomidina Kalceks
Mantener este medicamento fuera de la vista y del alcance de los niños.
Este medicamento no requiere condiciones especiales de conservación.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
6. Contenido del envase e información adicional
Composición de Dexmedetomidina Kalceks
- El principio activo es la dexmedetomidina. Cada ml de concentrado contiene hidrocloruro de dexmedetomidina equivalente a 100 microgramos de dexmedetomidina.
- Los demás componentes son cloruro de sodio y agua para preparaciones inyectables.
Cada ampolla de 2 ml contiene 200 microgramos de dexmedetomidina (como hidrocloruro).
Cada vial de 4 ml contiene 400 microgramos de dexmedetomidina (como hidrocloruro).
Cada vial de 10 ml contiene 1000 microgramos de dexmedetomidina (como hidrocloruro).
La concentración de la solución final tras la dilución debe ser de 4 microgramos/ml o de 8 microgramos/ml.
Aspecto de Dexmedetomidina Kalceks y contenido del envase
Concentrado para solución para perfusión (concentrado estéril).
El concentrado es una solución transparente e incolora o amarillenta.
Dexmedetomidina Kalceks se produce en ampollas de vidrio incoloro de 2 ml y viales de vidrio incoloro de 4 ml ó 10 ml.
Tamaños de envase:
5 ó 25 ampollas de 2 ml
1 ó 4 viales de 4 ml
1 ó 4 viales de 10 ml
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
AS KALCEKS
Krustpils iela 71E, Riga, LV‑1057, Letonia
Tel.: +371 67083320
E-mail: [email protected]
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Grindeks Kalceks España, S.L.
c/ José Abascal, 58 2º dcha
28003 Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Dinamarca Dexmedetomidin Kalceks
Austria Dexmedetomidin Kalceks 100 Mikrogramm/ml Konzentrat zur Herstellung einer Infusionslösung
Bélgica Dexmedetomidine Kalceks 100 microgrammes/ml solution à diluer pour perfusion Dexmedetomidine Kalceks 100 microgram/ml concentraat voor oplossing voor infusie
Dexmedetomidine Kalceks 100 Mikrogramm/ml Konzentrat zur Herstellung einer Infusionslösung
Bulgaria ??????????????? ??????? 100 ??????????/ml ?????????? ?? ?????????? ???????
Croacia Deksmedetomidin Kalceks 100 mikrograma/ml koncentrat za otopinu za infuziju
Republica checa Dexmedetomidine Kalceks
Estonia Dexmedetomidine Kalceks
Finlandia Dexmedetomidine Kalceks
Francia DEXMEDETOMIDINE KALCEKS 100 microgrammes/mL, solution à diluer pour perfusion
Alemania Dexmedetomidin Ethypharm 100 Mikrogramm/ml Konzentrat zur Herstellung einer Infusionslösung
Hungría Dexmedetomidine Kalceks 100 mikrogramm/ml koncentrátum oldatos infúzióhoz
Irlanda Dexmedetomidine 100 micrograms/ml concentrate for solution for infusion
Italia Dexmedetomidina Kalceks
Letonia Dexmedetomidine Kalceks 100 mikrogrami/ml koncentrats infuziju škiduma pagatavošanai
Lituania Dexmedetomidine Kalceks 100 mikrogramu/ml koncentratas infuziniam tirpalui
Noruega Dexmedetomidine Kalceks
Polonia Dexmedetomidine Kalceks
Portugal Dexmedetomidina Kalceks
Rumania Dexmedetomidina Kalceks 100 micrograme/ml concentrat pentru solutie perfuzabila
Eslovaquia Dexmedetomidine Kalceks 100 mikrogramov/ml infúzny koncentrát
Eslovenia Deksmedetomidin Kalceks 100 mikrogramov/ml koncentrat za raztopino za infundiranje
España Dexmedetomidina Kalceks 100 microgramos/ml concentrado para solución para perfusión EFG
Suecia Dexmedetomidine Kalceks
Los países bajos Dexmedetomidine Kalceks 100 microgram/ml concentraat voor oplossing voor infusie
Fecha de la última revisión de este prospecto:Septiembre 2023.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
DexmedetomidinaKalceks100microgramos/ml concentrado parasolución para perfusión EFG
Forma de administración
Dexmedetomidina Kalceks se debe administrar por profesionales sanitarios expertos en el manejo de pacientes que requieren cuidados intensivos o en el manejo de la anestesia en pacientes en el quirófano. Se debe administrar únicamente como perfusión diluida intravenosa empleando un dispositivo para perfusión controlada.
Preparación de la solución
Dexmedetomidina Kalceks se puede diluir en glucosa 50 mg/ml (5%), solución Ringer, lactato de Ringer, manitol o solución inyectable de cloruro de sodio 9 mg/ml (0,9%) para lograr la concentración requerida de 4 microgramos/ml o de 8 microgramos/ml antes de la administración. Ver más abajo en forma de tabla los volúmenes necesarios para preparar la perfusión.
En caso de que se requiera una concentración de 4 microgramos/ml:
Volumen de Dexmedetomidina Kalceks 100 microgramos/ml concentrado para solución para perfusión EFG | Volumen del diluyente | Volumen total de perfusión |
2 ml | 48 ml | 50 ml |
4 ml | 96 ml | 100 ml |
10 ml | 240 ml | 250 ml |
20 ml | 480 ml | 500 ml |
En caso de que se requiera una concentración de 8 microgramos/ml:
Volumen de Dexmedetomidina Kalceks 100 microgramos/ml concentrado para solución para perfusión EFG | Volumen del diluyente | Volumen total de perfusión |
4 ml | 46 ml | 50 ml |
8 ml | 92 ml | 100 ml |
20 ml | 230 ml | 250 ml |
40 ml | 460 ml | 500 ml |
La solución se debe agitar suavemente para mezclar bien.
Este medicamento se debe inspeccionar visualmente para detectar partículas y coloración antes de su administración.
Este medicamento ha demostrado ser compatible cuando se administra con los siguientes fluidos y medicamentos intravenosos:
Lactato de Ringer, solución de glucosa al 5%, solución inyectable de cloruro de sodio 9 mg/ml (0,9%), manitol 200 mg/ml (20%), tiopental sódico, etomidato, bromuro de vecuronio, bromuro de pancuronio, succinilcolina, besilato de atracurio, cloruro de mivacurio, bromuro de rocuronio, bromuro de glicopirrolato, fenilefrina HCl, sulfato de atropina, dopamina, noradrenalina, dobutamina, midazolam, sulfato de morfina, citrato de fentanilo, y un sustituto del plasma.
Incompatibilidades
Los estudios de compatibilidad han demostrado potencial para la adsorción de dexmedetomidina a algunos tipos de caucho natural. Aunque dexmedetomidina se dosifica en función del efecto, se recomienda utilizar componentes con juntas de caucho sintético o natural recubiertas.
Periodo de validez tras la dilución
Se ha demostrado la estabilidad química y física de la perfusión diluida durante 36 horas a 25°C y en condiciones refrigeradas (2°C ? 8°C).
Desde un punto de vista microbiológico, el producto debe utilizarse inmediatamente. Si no se usa de inmediato, los tiempos y condiciones de almacenamiento previo a su uso son responsabilidad del usuario y no deberán ser normalmente superiores a 24 horas entre 2° y 8°C, a no ser que la dilución se haya realizado en condiciones asépticas controladas y validadas.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DEXMEDETOMIDINA KALCEKS 100 MICROGRAMOS/ML CONCENTRADO PARA SOLUCION PARA PERFUSION EFGForma farmacéutica: INYECTABLE PERFUSION, 100 microgramos dexmedetomidina/mlPrincipio activo: DexmedetomidinaFabricante: Orion CorporationRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 µg/mlPrincipio activo: DexmedetomidinaFabricante: Orion CorporationRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 microgramos dexmedetomidina/mlPrincipio activo: DexmedetomidinaFabricante: Orion CorporationRequiere receta
Médicos online para DEXMEDETOMIDINA KALCEKS 100 MICROGRAMOS/ML CONCENTRADO PARA SOLUCION PARA PERFUSION EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DEXMEDETOMIDINA KALCEKS 100 MICROGRAMOS/ML CONCENTRADO PARA SOLUCION PARA PERFUSION EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















