
COMBIGAN 2 mg/ml + 5 mg/ml COLIRIO EN SOLUCION

Cómo usar COMBIGAN 2 mg/ml + 5 mg/ml COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
COMBIGAN 2 mg/ml + 5 mg/ml colirio en solución.
Tartrato de brimonidina y timolol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamentoporque contiene información importante para usted.
|
Contenido del prospecto
- Qué es COMBIGAN y para qué se utiliza
- Qué necesita saber antes de empezar a usar COMBIGAN
- Cómo usar COMBIGAN
- Posibles efectos adversos
- Conservación de COMBIGAN
- Contenido del envase e información adicional
1. Qué es Combigan y para qué se utiliza
COMBIGAN es un colirio que se utiliza para el control del glaucoma. Contiene dos medicamentos (brimonidina y timolol) que ambos reducen la presión elevada en el ojo. Brimonidina pertenece a un grupo de medicamentos llamados agonistas de los receptores alfa-2 adrenérgicos. Timolol pertenece a un grupo de medicamentos llamados beta-bloqueantes.
COMBIGAN se receta para disminuir la presión elevada en el interior del ojo cuando el uso de colirios beta bloqueantes no es suficiente.
Su ojo contiene un líquido acuoso claro que alimenta el interior del ojo. Continuamente se elimina líquido hacia el exterior del ojo y se genera nuevo líquido para reponerlo. Si el líquido no puede salir al exterior del ojo lo suficientemente deprisa, la presión dentro del ojo aumenta y podría con el tiempo dañar su visión. COMBIGAN actúa reduciendo la producción de líquido y aumentando la cantidad de líquido que sale. Esto reduce la presión dentro del ojo mientras que se sigue generando nuevo líquido.
2. Qué necesita saber antes de empezar a usar COMBIGAN
No useCOMBIGAN colirio en solución:
- Si es usted alérgico(hipersensible) a tartrato de brimonidina, timolol, beta-bloqueanteso a cualquiera de los demás componentes deeste medicamento (incluidos en la sección 6). Los síntomas de una reacción alérgica pueden incluir hinchazón de la cara, labios y garganta, silbidos, sensación de debilidad, dificultad para respirar, picor o enrojecimiento alrededor del ojo.
- Si padece o ha padecido problemas respiratorios como asma, bronquitis obstructiva crónica severa(enfermedad pulmonar grave que puede causar sibilancias, dificultad para respirar y/o tos constante)
- Si padece de problemas cardiacos como ritmo cardiaco lento, insuficiencia cardiaca, trastornos de los latidos del corazón(a no ser que esté controlado con marcapasos) Si está tomando inhibidores de la monoaminooxidasa (MAO) o algunos otros medicamentos antidepresores
Si cree que cualquiera de estos puntos son aplicables para usted, no use COMBIGAN hasta que haya consultado de nuevo con el médico.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar COMBIGAN,
- si padece o ha padecido de
- enfermedad cardiaca coronaria (los síntomas pueden incluir dolor u opresión en el pecho, dificultad para respirar o asfixia), insuficiencia cardiaca, presión arterial disminuida
- trastornos del ritmo cardiaco, como frecuencia cardiaca baja
- enfermedad circulatoria periférica (como enfermedad de Raynaud o el síndrome de Raynaud)
- diabetes, ya que timolol puede enmascarar los signos y síntomas de los niveles bajos de azúcar en la sangre hiperactividad de la glándula tiroidea, ya que timolol puede enmascarar los signos y síntomas.
- problemas renales o hepáticos
- tumor de la glándula adrenal
- cirugía ocular para reducir la presión en el ojo
- si sufre o ha sufrido de cualquier alergia (p.ej. fiebre del heno, eczema) o una reacción alérgica grave tenga presente que puede que sea necesario aumentar la dosis de adrenalina que se usa normalmente para controlar una reacción grave.
- advierta a su médico que está utilizando COMBIGAN, antes de someterse a una operación, ya que timolol puede cambiar los efectos de algunos medicamentos utilizados durante la anestesia.
Niños y adolescentes
COMBIGAN no se debe utilizar en niños de menos de 2 años ni generalmente en niños de entre 2 y 17 años.
Otros medicamentos y COMBIGAN
COMBIGAN puede afectar o ser afectado por otros medicamentos que esté utilizando, incluyendo otros colirios para el tratamiento de glaucoma.
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento, incluso medicamentos para cualquier otra enfermedad, aunque no estén relacionados con su problema ocular, e incluso los adquiridos sin receta médica.
Hay una serie de medicamentos que pueden interferir con COMBIGAN, por lo que es muy importante que le diga a su médico si está tomando:
- Analgésicos
- Medicamentos para ayudarle a dormir o para la ansiedad
- Medicamentos para tratar la presión arterial elevada (hipertensión)
- Medicamentos para alteraciones del corazón (por ejemplo latido anormal) tales como beta-bloqueantes, digoxina o quinidina (usada para tratar problemas cardiacos y algunos tipos de malaria)
- Medicamentos para tratar la diabetes o aumento de azúcar en la sangre
- Medicamentos para la depresión como fluoxetina y paroxetina
- Otros colirios utilizados para disminuir la presión elevada en el ojo (glaucoma)
- Medicamentos para tratar reacciones alérgicas severas
- Medicamentos que afecten a alguna de las hormonas de su organismo, como adrenalina y dopamina
- Medicamentos que afecten a los músculos de sus vasos sanguíneos
- Medicamentos para tratar el ardor de estómago o úlceras en el estómago
Si cambia la dosis de alguno de los medicamentos que tome o si consume alcohol de forma habitual, debe decírselo a su médico.
Si va a ser sometido a anestesia, debe decir a su médico o dentista que está usando COMBIGAN.
Embarazo y Lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No utilice COMBIGAN si usted está embarazada a menos que su médico lo considere necesario.
No utilice COMBIGAN si está en período de lactancia. Timolol puede pasar a la leche. Consulte a su médico antes de utilizar cualquier medicamento durante la lactancia.
Conducción y uso de maquinaria
COMBIGAN puede causar somnolencia, cansancio o visión borrosa en algunos pacientes. No conduzca o maneje herramientas o máquinas hasta que los síntomas hayan desaparecido. Si nota algún problema, hable con su médico.
Combigancontiene cloruro de benzalconio
Este medicamento contiene 0,25 mg de cloruro de benzalconio en cada 5 ml de solución, equivalente a 0,05 mg/ml.
El cloruro de benzalconio se puede absorber por las lentes de contacto blandas alterando su color. Retirar las lentes de contacto antes de usar este medicamento y esperar 15 minutos antes de volver a colocarlas.
El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la córnea (capa transparente de la zona frontal del ojo). Consulte a su médico si siente una sensación extraña, escozor o dolor en el ojo después de usar este medicamento.
COMBIGAN contiene fosfatos:
Este medicamento contiene 52,9 mg de fosfatos en cada ml de solución, equivalente a 10,58 mg/ml.
Si sufre de daño grave en la córnea (la capa trasparente de la parte frontal del ojo) el tratamiento con fosfatos, en casos muy raros, puede provocar visión borrosa por acumulación de calcio.
3. Cómo usar COMBIGAN
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico. No se debe utilizar COMBIGAN en niños menores de 2 años y no se recomienda su uso habitual en niños y adolescentes (entre 2 y 17 años).
La dosis recomendada es una gota de COMBIGAN , dos veces al día, con unas 12 horas de diferencia. No cambie la dosis o deje de aplicarla sin consultar a su médico.
Si usa COMBIGAN junto con otros colirios, espere al menos cinco minutos entre su aplicación y la de los demás colirios.
Instrucciones de uso
No debe utilizar el envase si el precinto de seguridad está roto cuando abra el producto por primera vez.
Lávese las manos antes de abrir el envase. Incline la cabeza hacia atrás y mire hacia el techo.
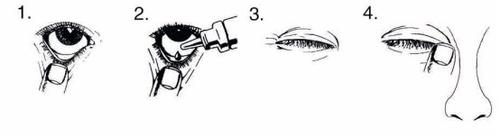
- Tire suavemente hacia abajo del párpado inferior hasta que quede un pequeño hueco.
- Invierta el frasco y apriételo para dejar salir unagota en cada ojo que necesite tratamiento.
- Suelte el párpado inferior, y cierre el ojo
- Mantenga el ojo cerrado y apriete con su dedo la parte del lagrimal (donde el ojo se junta con la nariz) durante dos minutos. Esto ayuda a impedir que COMBIGAN pase al resto del organismo.
Si la gota cae fuera del ojo, inténtelo de nuevo.
Para ayudar a prevenir infecciones, evite que la punta del envase toque el ojo, ni ninguna otra superficie. Cierre el envase inmediatamente después de usarlo.
Si usa másCOMBIGANdel que debiera
Adultos
Si usa más COMBIGAN del que debiera, es poco probable que le produzca algún daño. Póngase la siguiente gota a la hora habitual. Si está preocupado, hable con su médico o farmacéutico
Bebés y niños
Se han dado algunos casos de sobredosis en bebes y niños tratados con brimonidina (uno de los componentes de COMBIGAN) como parte del tratamiento para glaucoma. Se observó somnolencia, flacidez, baja temperatura corporal, palidez y dificultades respiratorias. Si esto ocurriera, contacte con su médico inmediatamente.
Adultos y niños
En caso de ingestión accidental contacte con su médico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicología, teléfono 91-562 04 20.
Si olvidó usarCOMBIGAN,
Si olvidó usar COMBIGAN, utilice una sola gota en cada ojo que necesite tratamiento en cuanto se acuerde, y luego vuelva a su rutina regular. No duplique la dosis para compensar las dosis olvidadas.
Si interrumpe el tratamiento conCOMBIGAN
Para que COMBIGAN actúe adecuadamente lo debe utilizar todos los días.
Si tiene alguna pregunta más sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si nota cualquiera de los siguientes efectos adversos, por favor contacte con su médico inmediatamente:
- Fallo cardíaco (es decir dolor de pecho) o latidos de corazón irregulares
- Aumento o disminución del ritmo del corazón o presión arterial baja
Se pueden observar los siguientes efectos adversos con COMBIGAN
Que afectan al ojo
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Enrojecimiento de los ojos, sensación de ardor
Frecuentes (pueden afectar hasta a 1 de cada 10 personas):
- Escozor o dolor en el ojo
- Reacción alérgica en el ojo o en la piel que lo rodea
- Pequeñas rupturas en la superficie del ojo (con o sin inflamación)
- Hinchazón, enrojecimiento de los párpados
- Irritación ocular o sensación de cuerpo extraño
- Picor del ojo y párpado
- Folículos o manchas blancas en la capa que cubre la superficie del ojo, a través de la que vemos
- Alteración de la visión
- Lagrimeo
- Sequedad ocular
- Ojos pegajosos
Poco frecuentes (pueden afectar hasta a 1 de cada 100 personas):
- Dificultad para ver con claridad
- Hinchazón o inflamación la capa que cubre la superficie del ojo, a través de la que vemos
- Vista cansada
- Sensibilidad a la luz
- Dolor en el párpado
- Blanqueamiento de la capa que cubre la superficie del ojo, a través de la que vemos
- Hinchazón o áreas inflamadas bajo la superficie del ojo
- Moscas volantes enfrente de los ojos
Frecuencia no conocida (la frecuencia no puede estimarse a partir de los datos disponibles):
- Visión borrosa
Que afectan al cuerpo:
Frecuentes (pueden afectar hasta a 1 de cada 10 personas):
- Presión sanguínea elevada
- Depresión
- Somnolencia
- Dolor de cabeza
- Sequedad en la boca
- Debilidad general
Poco frecuentes (pueden afectar hasta a 1 de cada 100 personas):
- Insuficiencia cardiaca
- Ritmo cardiaco irregular
- Sensación de mareo
- Desmayo
- Sequedad en la nariz
- Alteración del sabor
- Nauseas
- Diarrea
Frecuencia no conocida la frecuencia no puede estimarse a partir de los datos disponibles):
- Aumento o disminución del ritmo cardíaco
- Presión sanguínea baja
- Enrojecimiento en la cara
Algunos de estos efectos pueden ser debidos a una reacción alérgica a alguno de los componentes del producto.
Se han observado reacciones adversas adicionales con brimonidina o timolol, y por lo tanto, podrían presentarse con COMBIGAN.
Las siguientes reacciones adversas se han observado adicionalmente con brimonidina:
- Inflamación en el ojo, pupilas pequeñas, dificultad para dormir, síntomas similares a los del resfriado, dificultad para respirar, síntomas del estómago y de la digestión, reacciones alérgicas generales, reacciones cutáneas incluyendo enrojecimiento, hinchazón de la cara, picor, erupción cutánea y dilatación de los vasos sanguíneos
Como cualquier otro medicamento aplicado en los ojos, COMBIGAN (brimonidina/timolol) se absorbe en la sangre. La absorción de timolol, un componente betabloqueante de COMBIGAN, puede causar reacciones adversas similares a las que se observan con los betabloqueantes intravenosos y/o orales. La incidencia de reacciones adversas tras la administración oftálmica tópica es menor que con medicamentos orales o inyectados.
Las siguientes reacciones adversas se han observado con el uso de betabloqueantes para tratar enfermedades oculares:
- Reacciones alérgicas generalizadas, incluyendo hinchazón bajo la piel (puede ocurrir en áreas como la cara o las extremidades, y puede llegar a obstruir la vía aérea y causar dificultad para tragar o respirar), urticaria (erupción con picor), erupción cutánea localizada o generalizada, picor, reacción alérgica repentina que puede poner en peligro la vida
- Nivel bajo de glucosa en sangre
- Dificultad para dormir (insomnio), pesadillas, pérdida de memoria, alucinación
- Ictus, reducción del aporte sanguíneo al cerebro, empeoramiento de los síntomas de miastenia gravis (trastorno muscular), sensaciones extrañas (como hormigueo)
- Inflamación en la córnea, desprendimiento de la capa de la retina que contiene los vasos sanguíneos tras cirugía de filtración, que puede causar alteraciones de la visión, menor sensibilidad corneal, erosión corneal (daño en la capa frontal del globo ocular), párpado caído (el ojo aparece medio cerrado), visión doble
- Dolor en el pecho, edema (aumento de líquido), cambios en la velocidad o ritmo del latido cardiaco, algún trastorno en el ritmo cardiaco, infarto, insuficiencia cardiaca
- Fenómeno de Raynaud, manos y pies fríos
- Constricción de las vías aéreas del pulmón (principalmente en pacientes con una enfermedad preexistente) dificultad para respirar, tos
- Indigestión, dolor abdominal, vómitos
- Pérdida de cabello, erupción cutánea de color blanco-plateado (erupción psoriasiforme) o empeoramiento de la psoriasis, erupción cutánea
- Dolor muscular no causado por ejercicio
- Disfunción sexual, disminución de la libido
- Debilidad muscular/cansancio
Otros efectos adversos notificados con colirios que contienen fosfato:
Si sufre de daño grave en la córnea (la capa trasparente de la parte frontal del ojo) el tratamiento con fosfatos, en casos muy raros, puede provocar visión borrosa por acumulación de calcio.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de COMBIGAN
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar el envase en el embalaje exterior para protegerlo de la luz
No debe usar más de un envase al mismo tiempo.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el envase después de EXP. La fecha de caducidad es el último día del mes que se indica.
Debe desechar el envase cuatro semanas después de abrirlo por primera vez, incluso si todavía quedan algunas gotas. Esto ayudará a prevenir infecciones. Para ayudarle a recordar, escriba la fecha en que lo abrió en el espacio correspondiente en la caja.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico como deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de COMBIGAN
- Los principios activos son tartrato de brimonidina y timolol.
- Un mililitro de solución contiene 2 miligramos de tartrato de brimonidina y maleato de timolol equivalente a 5 miligramos de timolol.
- Los demás componentes son: cloruro de benzalconio (como conservante), fosfato sódico monobásico monohidratado, fosfato sódico dibásico heptahidratado y agua purificada. Se pueden añadir pequeñas cantidades de ácido clorhídrico o hidróxido sódico para ajustar el pH (determinación de la acidez o alcalinidad de la solución).
Aspecto de COMBIGAN y contenido del envase
COMBIGAN es un colirio en solución clara ligeramente amarillo verdosa en un envase de plástico con tapón a rosca. Cada envase está lleno hasta aproximadamente la mitad y contiene 5 ml de solución. Hay cajas disponibles conteniendo 1 o 3 envases. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
AbbVie Spain, S.L.U.
Avenida de Burgos 91,
28050 Madrid
España
Responsable de la fabricación:
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport, Co Mayo
Irlanda
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria | Combigan 2 mg/ml + 5 mg/ml Augentropfen |
Bélgica | Combigan 2 mg/ml + 5 mg/ml oogdruppels, oplossing |
Bulgaria | ???????? 2 mg/ml + 5 mg/ml ????? ?? ???, ??????? Combigan 2 mg/ml + 5 mg/ml eye drops, solution |
República Checa | COMBIGAN 2mg/ml + 5 mg/ml ocní kapky, roztok |
Croacia | Combigan 2 mg/ml + 5 mg/ml kapi za oko, otopina |
Dinamarca | Combigan 2 mg/ml + 5 mg/ml øjendråber, opløsning |
Estonia | Combigan, 2 mg/5 mg/ml silmatilgad, lahus |
Finlandia | Combigan 2 mg/ml + 5 mg/ml silmätipat, liuos |
Francia | COMBIGAN 2 mg/ml + 5 mg/ml, collyre en solution |
Alemania | Combigan 2 mg/ml + 5 mg/ml Augentropfen |
Grecia | COMBIGAN οφθαλμικ?ς σταγ?νες, δι?λυμα, (0,2 + 0,5)% |
Hungría | COMBIGAN 2 mg/ml+5 mg/ml oldatos szemcsepp |
Islandia | Combigan 2 mg/ml + 5 mg/ml augndropar, lausn |
Irlanda | Combigan 2 mg/ml + 5 mg/ml eye drops, solution |
Italia | COMBIGAN 2 mg/ml + 5 mg/ml collirio, soluzione |
Letonia | Combigan2 mg/5 mg/ml acu pilieni, škidums |
Lituania | Combigan 2 mg/5 mg/ml akiu lašai (tirpalas) |
Luxemburgo | Combigan 2 mg/ml + 5 mg/ml, collyre en solution |
Holanda | Combigan 2 mg/ml + 5 mg/ml, oogdruppels, oplossing |
Noruega | Combigan 2 mg/ml + 5 mg/ml øyedråper, oppløsning |
Polonia | Combigan krople do oczu, roztwór, 2 mg/ml + 5 mg/ml |
Portugal | Combigan 2 mg/ml + 5 mg/ml colírio, solução |
Rumanía | Combigan 2 mg/ml + 5 mg/ml picaturi oftalmice, solutie |
Eslovaquia | COMBIGAN® 2 mg/ml + 5 mg/ml ocná roztoková instilácia |
Eslovenia | COMBIGAN® 2 mg/ml + 5 mg/ml kapljice za oko, raztopina |
España | Combigan 2mg/ml + 5 mg/ml colirio en solución |
Suecia | Combigan 2 mg/ml + 5 mg/ml ögondroppar, lösning |
Reino Unido | Combigan 2 mg/ml + 5 mg/ml eye drops, solution |
Fecha de la última revisión de este prospecto: 11/ 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia15.67 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a COMBIGAN 2 mg/ml + 5 mg/ml COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 10 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml+5mg/mlPrincipio activo: timolol, combinationsFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para COMBIGAN 2 mg/ml + 5 mg/ml COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de COMBIGAN 2 mg/ml + 5 mg/ml COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















