БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці

Запитайте лікаря про рецепт на БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці

Інструкція із застосування БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці
Вступ
Опис: інформація для користувача
Boostrix Polio, суспензія для ін'єкцій у попередньо заповненому шприці
Вакцина проти дифтерії, тетаносу, коклюшу (ацикуларний компонент) та поліомієліту (інактивований) (адсорбована, вміст антигену зменшено)
Прочитайте уважно весь опис перед тим, як ви або ваша дитина отримає цю вакцину, оскільки вона містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь сумніви, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цю вакцину призначено лише вам або вашій дитині, і не слід давати її іншим людям.
- Якщо ви або ваша дитина відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке Boostrix Polio і для чого вона використовується
- Що потрібно знати перед тим, як ви або ваша дитина отримає Boostrix Polio
- Як використовувати Boostrix Polio
- Можливі побічні ефекти
- Збереження Boostrix Polio
- Вміст упаковки та додаткова інформація
1. Що таке Boostrix Polio і для чого вона використовується
Boostrix Polio - це вакцина, призначена для ревакцинації дітей від 3 років, підлітків і дорослих для профілактики чотирьох захворювань: дифтерії, тетаносу (ригідності щелепи), коклюшу та поліомієліту (поліо). Вакцина діє шляхом допомоги організму у виробленні власного захисту (антитіл) проти цих захворювань.
- Дифтерія:Дифтерія переважно впливає на дихальні шляхи та іноді на шкіру. Зазвичай, дихальні шляхи запалюються (збільшуються) та викликають серйозні труднощі з диханням та іноді асфіксію. Бактерія також виділяє токсин (отруту), який може викликати неврологічні ушкодження, проблеми з серцем та навіть смерть.
- Тетанос (ригідність щелепи):Бактерія тетаносу проникає в організм через порізи, подряпини або рани на шкірі. Рани, які особливо схильні до інфекції, - це опіки, переломи, глибокі рани або рани, забруднені брудом, пилом, кінським гноєм або дерев'яними щепками. Бактерія виділяє токсин (отруту), який може викликати м'язову ригідність, болісні м'язові спазми, конвульсії та навіть смерть. М'язові спазми можуть бути настільки сильними, що викликають переломи хребта.
- Коклюш:Коклюш - це високоінфекційне захворювання. Захворювання впливає на дихальні шляхи та викликає сильні кашлі, які можуть перешкоджати нормальному диханню. Кашель часто супроводжується "вільним" звуком, звідси походить назва захворювання. Кашель може тривати 1-2 місяці або довше. Також може викликати інфекції вуха, бронхіт, який може тривати довго, пневмонію, конвульсії, ушкодження мозку та навіть смерть.
- Поліомієліт(поліо): Поліомієліт, іноді званий просто "поліо", - це інфекція, викликана вірусом, який може мати різні ефекти. Часто викликає легке захворювання, але в деяких людей викликає постійне ушкодження або навіть смерть. У найтяжчій формі інфекція поліо викликає параліч м'язів (м'язи не можуть рухатися), включаючи м'язи, необхідні для дихання та ходьби. Уражені захворюваннями кінцівки можуть деформуватися болісно.
Жоден з компонентів вакцини не може викликати дифтерію, тетанос, коклюш або поліомієліт.
Використання Boostrix Polio під час вагітності допоможе захистити вашу дитину від коклюшу в перші місяці життя, до того як вона отримає первинну імунізацію.
2. Що потрібно знати перед тим, як ви або ваша дитина отримає Boostrix Polio
Не слід вводити Boostrix Polio:
- якщо ви або ваша дитина раніше мали будь-яку алергічну реакцію на Boostrix Polio або на будь-який інший компонент цієї вакцини (вказані в розділі 6), або на неоміцин, поліміксин (антібіотики) або формальдегід. Ознаки алергічної реакції можуть включати висипку, свербіж, труднощі з диханням та набряк обличчя або язика
- якщо ви або ваша дитина раніше мали алергічну реакцію на будь-яку вакцину проти дифтерії, тетаносу, коклюшу або поліомієліту
- якщо ви або ваша дитина раніше мали проблеми з нервовою системою (енцефалопатію) у 7 днів після попередньої вакцинації вакциною проти коклюшу
- якщо ви або ваша дитина раніше мали тимчасове зниження кількості тромбоцитів у крові (що збільшує ризик кровотечі або появи синяків) або проблеми з мозком або нервовою системою після попередньої вакцинації вакциною проти дифтерії і/або тетаносу
- якщо ви або ваша дитина мають серйозну інфекцію з високою температурою (вище 38°C). Легка інфекція не повинна бути проблемою, але спочатку проконсультуйтеся з вашим лікарем.
Попередження та обережність
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як ви або ваша дитина отримає Boostrix Polio:
- якщо ви або ваша дитина мали будь-які проблеми після попереднього введення Boostrix Polio або іншої вакцини проти коклюшу, особливо:
- Ліхорадка (вище 40°C) у 48 годин після вакцинації
- Колапс або стан, подібний до "шоку", у 48 годин після вакцинації
- Нескінченний плач, триваліший за 3 години, який виник у 48 годин після вакцинації
- Конвульсії, з або без ліхорадки, які виникли у 3 дні після вакцинації
- якщо ваша дитина має недіагностоване або прогресуюче захворювання мозку або епілепсію, яку не контролюють. Вакцину слід вводити після контролю захворювання
- якщо ви або ваша дитина маєте проблеми з кровотечею або легко отримуєте синяки
- якщо ви або ваша дитина маєте схильність до конвульсій через ліхорадку або якщо є сімейна історія цього
- якщо ви або ваша дитина маєте постійні проблеми з імунною системою через будь-яку причину (включаючи інфекцію ВІЛ). Ви або ваша дитина можете отримати Boostrix Polio, але захист проти інфекцій після вакцинації не буде таким же хорошим, як у пацієнтів з адекватними імунними реакціями на інфекції.
Перед або після будь-якої ін'єкції може виникнути знепритомнення (особливо у підлітків), тому слід повідомити вашого лікаря або медсестру, якщо ви або ваша дитина раніше мали знепритомнення після введення ін'єкції.
Як і всі вакцини, Boostrix Polio може не забезпечувати повний захист у всіх пацієнтів, які отримали вакцину.
Використання Boostrix Polio з іншими лікарськими засобами
Повідомте вашого лікаря або фармацевта, якщо ви або ваша дитина приймаєте, нещодавно приймали або можете приймати будь-які інші лікарські засоби або якщо нещодавно отримали іншу вакцину.
Boostrix Polio можна вводити одночасно з деякими вакцинами. Використовуйте окреме місце ін'єкції для кожної вакцини; місце ін'єкції буде різним.
Можливо, Boostrix Polio не забезпечить адекватної реакції, якщо ви або ваша дитина приймаєте лікарські засоби, які знижують ефективність імунної системи проти інфекцій.
Вагітність і лактація
Якщо ви вагітні або годуєте грудьми, вважаєте, що можете бути вагітною або плануєте завагітніти, проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як отримати цю вакцину.
Невідомо, чи проникає Boostrix Polio в грудне молоко. Ваш лікар повідомить вам про можливі ризики та переваги введення Boostrix Polio під час лактації.
Водіння транспортних засобів і використання машин
Малоймовірно, що Boostrix Polio матиме будь-який вплив на здатність водити транспортні засоби та використовувати машини.
Boostrix Polio містить неоміцин і поліміксин
Ця вакцина містить неоміцин і поліміксин (антібіотики). Будь ласка, повідомте вашого лікаря, якщо ви або ваша дитина мали алергічну реакцію на ці компоненти.
Boostrix Polio містить пара-амінобензойну кислоту, фенілаланін, натрій і калій
Boostrix Polio містить пара-амінобензойну кислоту. Вона може викликати алергічні реакції (можливо, затримані) та, винятково, бронхоспазм.
Ця вакцина містить 0,0298 мкг фенілаланіну в кожній одиниці дози. Фенілаланін може бути шкідливим у разі фенілкетонурії (ФКН), рідкого генетичного захворювання, при якому фенілаланін накопичується через те, що організм не може її правильно вивести.
Ця вакцина містить менше 1 ммоль натрію (23 мг) на одиницю дози; це означає, що вона практично "не містить натрію".
Ця вакцина містить калій, менше 1 ммоль (39 мг) на дозу; це означає, що вона практично "не містить калію".
3. Як використовувати Boostrix Polio
- Boostrix Polio вводиться як ін'єкція в м'яз.
- Вакцину ніколи не слід вводити внутрішньовенно.
- Ви або ваша дитина отримаєте одну ін'єкцію Boostrix Polio.
- Ваш лікар перевірить, чи ви або ваша дитина раніше отримували вакцини проти дифтерії, тетаносу, коклюшу та/або поліомієліту.
- Boostrix Polio можна використовувати у разі підозри на тетаносну інфекцію, хоча також слід вживати інші заходи для зменшення ризику прояву захворювання, наприклад, лікування рани та/або застосування тетаносної токсини.
- Ваш лікар порекомендує повторити вакцинацію.
4. Можливі побічні ефекти
Як і всі лікарські засоби, Boostrix Polio може викликати побічні ефекти, хоча не всі люди їх відчувають.
Як і всі ін'єкційні вакцини, можна відчувати серйозні алергічні реакції (анafilактичні та анафілактоїдні реакції) дуже рідко (до 1 з 10 000 доз вакцини). Це можна визначити за:
- Висипкою, свербіжем або пухирями,
- Набряком очей і обличчя,
- Труднощами з диханням або ковтанням,
- Раптовим зниженням артеріального тиску та втратою свідомості.
Ці реакції можуть виникнути до того, як ви покинете кабінет лікаря. Однак якщо ви помітите будь-які з цих симптомів у себе або вашої дитини, негайно зверніться до вашого лікаря.
Побічні ефекти, які виникли під час клінічних досліджень у дітей від 4 до 8 років
Дуже часті(можуть виникнути у понад 1 з 10 доз вакцини): біль, червоність та набряк в місці ін'єкції, сонливість.
Часті(можуть виникнути до 1 з 10 доз вакцини): ліхорадка вище 37,5°C (включаючи ліхорадку вище 39°C), кровотеча, свербіж та твердіння в місці ін'єкції, набряк кінцівки, яку влучили вакциною, відсутність апетиту, раздражливість, головний біль.
Нечасті(можуть виникнути до 1 з 100 доз вакцини): діарея, нудота, блювота, біль у животі, набряк залоз шиї, паху або пахвинної області (лімфаденопатія), проблеми з сном, апатія, сухість у горлі, втома.
Коадміністрування з вакцинами проти корі, краснухи, епідемічного паротиту (КРЕ) або корі, краснухи, епідемічного паротиту, вітряної віспи (КРЕВ) у дітей від 3 до 6 років
У дослідженнях, в яких Boostrix Polio вводили одночасно з вакцинами КРЕ або КРЕВ, часто повідомляли про висипку та інфекції верхніх дихальних шляхів (включаючи виділення з носа та біль у горлі). Ліхорадка, раздражливість, втома, відсутність апетиту та порушення травлення (включаючи діарею та блювоту) повідомлялися частіше (дуже часті) ніж у дослідженнях, в яких Boostrix Polio вводили окремо.
Побічні ефекти, які виникли під час клінічних досліджень у дорослих, підлітків та дітей від 10 років:
Дуже часті(можуть виникнути у понад 1 з 10 доз вакцини): біль, червоність та набряк в місці ін'єкції, втома, головний біль.
Часті(можуть виникнути до 1 з 10 доз вакцини): ліхорадка вище 37,5°C, синяк, свербіж, твердіння, оніміння з теплом в місці ін'єкції, біль у животі, нудота, блювота.
Нечасті(можуть виникнути до 1 з 100 доз вакцини): ліхорадка вище 39°C, набряк кінцівки, яку влучили вакциною, озноб, біль, головокружіння, біль у суглобах, біль у м'язах, свербіж, герпес на губах, набряк залоз шиї, паху або пахвинної області (лімфаденопатія), відсутність апетиту, оніміння або поколювання рук або ніг (парестезія), сонливість, астма.
Побічні ефекти, які виникли під час звичайного використання Boostrix Polio, і які не є специфічними для жодної вікової групи: колапс або втрата свідомості, набряк обличчя, губ, рота, язика або горла, який може викликати труднощі з ковтанням або диханням (ангіоневротичний набряк), конвульсії (з або без ліхорадки), кропив'янка (уртикарія), незвичайна слабкість (астенія).
Крім того, наступні побічні ефекти повідомлялися під час клінічних досліджень з Boostrix (вакциною ревакцинації GlaxoSmithKline проти дифтерії, тетаносу та коклюшу):
Побічні ефекти, які виникли у дітей від 4 до 8 років
Нечасті(можуть виникнути до 1 з 100 доз вакцини): порушення уваги, виділення з очей та повік із коростою (кон'юнктивіт), біль.
Побічні ефекти, які виникли у дорослих, підлітках та дітях від 10 років:
Дуже часті(можуть виникнути у понад 1 з 10 доз вакцини): загальне нездужання.
Часті(можуть виникнути до 1 з 10 доз вакцини): індуративні або абсцесні утворення в місці ін'єкції.
Нечасті(можуть виникнути до 1 з 100 доз вакцини): інфекція верхніх дихальних шляхів, біль у горлі та труднощі з ковтанням (фарингіт), втрата свідомості (синкоп), кашель, діарея, надмірне потіння (гіпергідроз), висипка, ригідність суглобів, ригідність м'язів та суглобів, симптоми, подібні до грипу, такі як ліхорадка, біль у горлі, виділення з носа, кашель та озноб.
Після введення вакцин проти тетаносу дуже рідко (до 1 з 10 000 доз вакцини) повідомлялися випадки тимчасового запалення нервів, яке викликає біль, слабкість та параліч кінцівок, та часто поширюється на груди та обличчя (синдром Гієна-Барре).
Повідомлення про побічні ефекти
Якщо ви або ваша дитина відчуваєте будь-які побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Також можна повідомити про них безпосередньо через систему моніторингу лікарських засобів України. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Збереження Boostrix Polio
Тримайте цю вакцину поза зоною досяжності дітей.
Не використовуйте цю вакцину після закінчення терміну придатності, вказаного на упаковці та на етикетці шприца після "CAD". Термін придатності - останній день місяця, який вказано.
Зберігайте у холодильнику (між 2°C та 8°C).
Не заморожуйте. Заморожування знищує вакцину.
Зберігайте в оригінальній упаковці, щоб захистити від світла.
Лікарські засоби не слід викидати у водопровідні труби чи сміттєві контейнери. Відкладайте упаковки та лікарські засоби, які вам не потрібні, у спеціальний контейнер для лікарських відходів у аптеці. Якщо у вас є сумніви, запитайте у вашого фармацевта, як позбутися упаковок та лікарських засобів, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Бустрикса Поліо
- Активні речовини:
Дифтерійний токсоїд1 не менше 2 Міжнародних одиниць (МО) (2,5 Лф)
Тетанічний токсоїд1 не менше 20 Міжнародних одиниць (МО) (5 Лф)
Антігени Bordetella pertussis
Пертусний токсоїд1 8 мікрограм
Філаментозна гемаглютинін1 8 мікрограм
Пертактин1 2,5 мікрограм
Інактивовані полiovirus
Тип 1 (штам Махоні)2 40 одиниць антигену D
Тип 2 (штам MEF-1)2 8 одиниць антигену D
Тип 3 (штам Сокет)2 32 одиниці антигену D
1 адсорбовані на гідроксиді алюмінію гідратованому (Al(OH)3) 0,3 міліграма Al3+
і фосфаті алюмінію (AlPO4) 0,2 міліграма Al3+
2 розмножені в клітинах Vero
Гідроксид алюмінію та фосфат алюмінію включені до вакцини як ад'юванти.
Ад'юванти - речовини, які включені до деяких вакцин для прискорення, покращення та/або подовження захисного ефекту вакцини.
- Інші компоненти: Середа 199 (містить амінокислоти (в тому числі фенілаланін), мінеральні солі (в тому числі натрій і калій), вітаміни (в тому числі пара-амінобензойна кислота) та інші речовини), хлорид натрію і вода для ін'єкційних препаратів.
Вигляд продукту та вміст упаковки
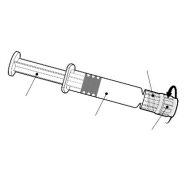
Ін'єкційна суспензія у попередньо заповненому шприці.
Бустрикс Поліо - це білий, легенько молочний рідина, яка подається у попередньо заповненому шприці (0,5 мл).
Бустрикс Поліо доступний у попередньо заповненому шприці з однією дозою з або без окремих голок; розміри упаковки 1 і 10.
Можливо, що тільки деякі розміри упаковок будуть реалізовані.
Власник дозволу на продаж та відповідальна особа за виробництво
Власник дозволу на продаж
GlaxoSmithKline, S.A.
P.T.M - C/ Severo Ochoa, 2
28760 Трес-Кантос
Мадрид
Тел: 900 202 700
Відповідальна особа за виробництво
GlaxoSmithKline Biologicals S.A.
Rue de l' Institut 89; 1330 Ріксансарт
Бельгія
Цей лікарський засіб дозволений в державах-членах Європейського економічного простору під наступними назвами:
Бустрикс Поліо:Бельгія, Болгарія, Чехія, Данія, Німеччина, Греція, Іспанія, Ісландія, Латвія, Литва, Люксембург, Угорщина, Нідерланди, Норвегія, Австрія, Польща, Португалія, Словенія, Фінляндія, Швеція
Бустрикс Поліо Лаг:Словаччина
Бустрикс Тетра:Франція
ІПВ-Бустрикс:Ірландія, Мальта
Поліо Бустрикс:Італія
Бустрикс-ІПВ:Румунія
Дата останнього перегляду цієї інструкції: квітень 2023
Інші джерела інформації
Детальна інформація про цей лікарський засіб доступна на сайті Іспанського агентства лікарських засобів і медичних продуктів (AEMPS) (http://www.aemps.gob.es/).
-------------------------------------------------------------------------------------------------------------------
Ця інформація призначена лише для фахівців галузі охорони здоров'я:
Перед використанням вакцини її слід залишити при кімнатній температурі та добреagitувати для отримання білої, мутної та однорідної суспензії. Перед введенням вакцини слід візуально оглянути її на наявність сторонніх частинок та/або зміни фізичного вигляду. У разі виявлення якихось із цих обставин не вводити вакцину.
Інструкції для попередньо заповненого шприця
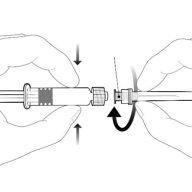
| Тримайте шприц за корпус, а не за поршень. Відкрутіть кришечку шприця, повертаючи її проти годинникової стрілки. |
| Для введення голки підключіть основу до адаптера луер-локта поверніть на чверть обертання за годинниковою стрілкою до відчуття блокування. Не витягуйте поршень із корпуса шприця. Якщо це трапиться, не вводьте вакцину. |
Видалення відходів:
Видалення невикористаного лікарського засобу та всіх матеріалів, які були в контакті з ним, здійснюється згідно з місцевими правилами.
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприціФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 0,5 млДіючі речовини: diphtheria-pertussis-poliomyelitis-tetanusВиробник: Glaxosmithkline S.A.Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 0,5 млДіючі речовини: diphtheria-pertussis-poliomyelitis-tetanusВиробник: Sanofi Winthrop IndustrieПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 1 дозаДіючі речовини: diphtheria-pertussis-poliomyelitis-tetanusВиробник: Sanofi Winthrop IndustrieПотрібен рецепт
Аналоги БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці у Польша
Аналог БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці у Украина
Лікарі онлайн щодо БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на БУСТРИКС ПОЛІО Ін'єкційна суспензія в попередньо заповненому шприці – за рішенням лікаря та згідно з місцевими правилами.