ABRYSVO POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar ABRYSVO POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Abrysvo polvo y disolvente para solución inyectable
vacuna frente al virus respiratorio sincitial (bivalente, recombinante)
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a recibir esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Abrysvo y para qué se utiliza
- Qué necesita saber antes de recibir Abrysvo
- Cómo se administra Abrysvo
- Posibles efectos adversos
- Conservación de Abrysvo
- Contenido del envase e información adicional
1. Qué es Abrysvo y para qué se utiliza
Abrysvo es una vacuna para prevenir la enfermedad pulmonar (de las vías respiratorias) causada por un virus llamado virus respiratorio sincitial (VRS). Abrysvo se administra a:
- mujeres embarazadas para proteger a sus bebés desde el nacimiento hasta los 6 meses de edad,
o
- personas de 18 años de edad y mayores.
El VRS es un virus habitual que, en la mayoría de los casos, causa síntomas leves parecidos a los de un resfriado, como dolor de garganta, tos o congestión nasal. Sin embargo, en los lactantes pequeños, el VRS puede causar problemas pulmonares graves. En adultos de edad avanzada y en personas con patologías crónicas, el VRS puede empeorar enfermedades como la enfermedad pulmonar obstructiva crónica (EPOC) y la insuficiencia cardíaca congestiva (ICC). El VRS puede dar lugar a hospitalización en casos graves y, en algunas circunstancias, puede ser mortal.
Cómo funciona Abrysvo
Esta vacuna ayuda al sistema inmune (las defensas naturales del organismo) a producir anticuerpos (sustancias en la sangre que ayudan al cuerpo a combatir infecciones) que protegen contra la enfermedad pulmonar causada por el VRS. En las mujeres embarazadas vacunadas entre las semanas 24 y 36 de embarazo, estos anticuerpos pasan al bebé a través de la placenta antes del nacimiento, lo que protege a los bebés cuando corren mayor riesgo de contraer el VRS.
2. Qué necesita saber antes de empezar a recibir Abrysvo
Abrysvo no se le debe administrar
- si es alérgico a los principios activos o a alguno de los demás componentes de esta vacuna (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de que le administren esta vacuna
- Si alguna vez ha sufrido una reacción alérgica grave o problemas respiratorios después de haber recibido alguna otra vacuna o después de que le administraran Abrysvo en el pasado.
- Si se siente nervioso por recibir la vacuna o alguna vez se ha desmayado después de una inyección. El desmayo puede ocurrir antes o después de cualquier inyección.
- Si sufre una infección con fiebre alta. Si este es el caso, la vacunación se pospondrá. No hay necesidad de retrasar la vacunación por una infección leve, como un resfriado, pero consulte primero con su médico.
- Si tiene un problema de sangrado o le salen moratones con facilidad.
- Si tiene un sistema inmunitario debilitado que puede impedirle obtener todos los beneficios de Abrysvo.
- Si tiene menos de 24 semanas de embarazo.
Si se cumple cualquiera de las situaciones mencionadas anteriormente (o no está seguro), consulte con su médico, farmacéutico o enfermero antes de que le administren Abrysvo.
Al igual que con cualquier vacuna, es posible que Abrysvo no proteja completamente a todos los que la reciben.
Niños y adolescentes
Abrysvo no está recomendado en niños y jóvenes menores de 18 años de edad, excepto durante el embarazo (ver más adelante la sección “Embarazo”).
Otros medicamentos y Abrysvo
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento o ha recibido recientemente alguna otra vacuna.
Abrysvo se puede administrar al mismo tiempo que la vacuna frente a la gripe o la COVID-19. Se recomienda un intervalo de al menos dos semanas entre la administración de Abrysvo y la vacuna frente al tétanos, difteria y tosferina acelular.
Embarazo y lactancia
Las mujeres embarazadas pueden recibir esta vacuna al final del segundo o tercer trimestre (semanas de 24 a 36). Si está en periodo de lactancia, consulte a su médico o enfermero antes de recibir esta vacuna.
Conducción y uso de máquinas
Es poco probable que Abrysvo afecte a su capacidad para conducir o utilizar máquinas.
Abrysvo contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
Abrysvo contiene polisorbato80
Una dosis de Abrysvo contiene 0,08 mg de polisorbato 80. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo se administra Abrysvo
Se le administrará una inyección de 0,5 ml en un músculo de la parte superior del brazo.
Si tiene alguna duda sobre el uso de Abrysvo, consulte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todas las vacunas, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Muy raros(pueden afectar hasta 1 de cada 10 000 personas)
- reacciones alérgicas graves: los signos de una reacción alérgica grave incluyen hinchazón de la cara, labios, lengua o garganta, , dificultad para respirar o tragar y mareos. Ver también la sección 2.
- síndrome de Guillain‑Barré (un trastorno neurológico que generalmente comienza con hormigueos y debilidad en las extremidades y puede evolucionar hasta la parálisis de parte o todo el cuerpo).
Informe a su médico inmediatamente si nota signos de estos efectos adversos graves.
Se han notificado los siguientes efectos adversos en mujeres embarazadas
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- dolor en el lugar de la inyección
- dolor de cabeza
- dolor muscular (mialgia).
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- enrojecimiento en el lugar de la inyección
- hinchazón en el lugar de la inyección.
Raros(pueden afectar hasta 1 de cada 1 000 personas)
- reacciones alérgicas como erupción o urticaria
- inflamación de las glándulas (linfadenopatía).
No se notificaron efectos adversos en lactantes nacidos de madres vacunadas.
Se han notificado los siguientes efectos adversos en personas de 18años de edad y mayores
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- cansancio (fatiga)
- dolor de cabeza
- dolor en el lugar de la inyección
- dolor muscular (mialgia).
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- dolor articular (artralgia)
- enrojecimiento en el lugar de la inyección
- hinchazón en el lugar de la inyección.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- fiebre (pirexia).
Raros(pueden afectar hasta 1 de cada 1 000 personas)
- reacciones alérgicas como erupción o urticaria
- inflamación de las glándulas (linfadenopatía)
- cardenales en el lugar de la inyección (hematoma)
- picor en el lugar de la inyección (prurito).
Muy raros(pueden afectar hasta 1 de cada 10 000 personas)
- reacciones alérgicas graves (ver Efectos adversos graves, más arriba)
- síndrome de Guillain-Barré (ver Efectos adversos graves, más arriba).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Abrysvo
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y la etiqueta después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC).
No congelar. Desechar si el envase se ha congelado.
Tras la reconstitución, Abrysvo se debe administrar inmediatamente o en las 4 horas siguientes si se conserva entre 15 ºC y 30 ºC. No congelar.
6. Contenido del envase e información adicional
Composición de Abrysvo
Los principios activos son:
Antígeno F estabilizado en prefusión del subgrupo A del VRS1,2 60 microgramos
Antígeno F estabilizado en prefusión del subgrupo B del VRS1,2 60 microgramos
(antígenos del VRS)
1glicoproteína F estabilizada en la conformación de prefusión.
2producido en células de ovario de hámster chino (OHC) mediante tecnología de ADN recombinante.
Los demás componentes son:
Polvo
- trometamol
- hidrocloruro de trometamol
- sacarosa
- manitol (E421)
- polisorbato 80 (E433)
- cloruro de sodio
- ácido clorhídrico
Disolvente
- agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Abrysvo se proporciona como:
- polvo blanco en un vial de vidrio
- disolvente en una jeringa precargada o un vial para disolver el polvo.
Después de disolver el polvo en el disolvente, la solución es transparente e incolora.
Abrysvo está disponible en:
- un envase con 1 vial de polvo, 1 jeringa precargada de disolvente, 1 adaptador del vial con 1 aguja o sin agujas (envase de 1 dosis);
- un envase con 5 viales de polvo, 5 jeringas precargadas de disolvente, 5 adaptadores del vial con 5 agujas o sin agujas (envase de 5 dosis);
- un envase con 10 viales de polvo, 10 jeringas precargadas de disolvente, 10 adaptadores del vial con 10 agujas o sin agujas (envase de 10 dosis);
- un envase con 5 viales de polvo y 5 viales de disolvente (envase de 5 dosis).
- un envase con 10 viales de polvo y 10 viales de disolvente (envase de 10 dosis).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Bélgica
Responsable de la fabricación
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Bélgica
Pfizer Ireland Pharmaceuticals
Grange Castle Business Park
Clondalkin, Dublin 22
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tél/Tel: + 32 (0)2 554 62 11 | Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: + 371 670 35 775 |
| Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Magyarország Pfizer KftTel: + 36 1 488 37 00 |
Danmark Pfizer ApS Tlf: + 45 44 20 11 00 | Malta Vivian Corporation Ltd. Tel: + 356 21344610 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Ελλ?δα Pfizer Ελλ?ς A.E.Τηλ.: +30 210 6785800 | Österreich Pfizer Corporation Austria Ges.m.b.H Tel: +43 (0)1 521 15-0 |
España Pfizer, S.L. Télf: +34 91 490 99 00 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
France Pfizer Tél +33 (0)1 58 07 34 40 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | RomâniaPfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: +1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, LjubljanaTel.: +386 (0)1 52 11 400 |
Ísland Icepharma hf. Simi: + 354 540 8000 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
K?προς Pfizer Ελλ?ς Α.Ε. (Cyprus Branch) Tηλ: +357 22817690 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Fecha de la última revisión de este prospecto:04/2025.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu
--------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
Trazabilidad
Con objeto de mejorar la trazabilidad de los medicamentos biológicos, el nombre y el número de lote del medicamento administrado deben estar claramente registrados.
Administración
Abrysvo únicamente se puede administrar por vía intramuscular.
El vial sin abrir es estable durante 5 días cuando se conserva a temperaturas de 8 ºC a 30 ºC. Al final de este período, Abrysvo debe utilizarse o desecharse. Esta información se utiliza para guiar a los profesionales sanitarios únicamente en caso de desviaciones temporales de la temperatura.
Conservación de la vacuna reconstituida
Abrysvo se debe administrar inmediatamente después de la reconstitución o en las 4 horas siguientes. Conserve la vacuna reconstituida entre 15 ºC y 30 ºC. No congele la vacuna reconstituida.
Se ha demostrado la estabilidad química y física en uso durante 4 horas entre 15 ºC y 30 ºC. Desde el punto de vista microbiológico, la vacuna se debe utilizar inmediatamente. Si no se usa inmediatamente, los tiempos y las condiciones de conservación antes de su utilización son responsabilidad del usuario.
Preparación para la administración
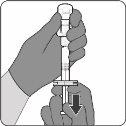
Para el uso del vial de antígenos para Abrysvo (polvo), jeringa precargada con disolvente y adaptador del vial
El polvo se debe reconstituir únicamente con el disolvente proporcionado en la jeringa precargada utilizando el adaptador del vial.
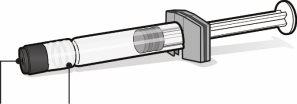
Jeringa precargada con disolvente para Abrysvo | Vial con antígenos para Abrysvo (polvo) | Adaptador del vial | |
|
|
| |
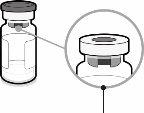
Capuchón de la jeringa | Adaptador Luer lock | Tapón del vial (sin la cápsula de cierre extraíble) |
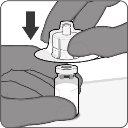
| Paso1. Coloque el adaptador del vial
|
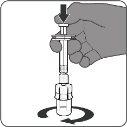
| Paso2. Reconstituya el componente en polvo (antígenos) para formar Abrysvo
|
| Paso3. Retire la vacuna reconstituida
|
La vacuna preparada es una solución transparente e incolora. Inspeccione visualmente la vacuna para detectar partículas grandes y cambios de color antes de la administración. No la use si observa partículas grandes o cambios de color.
Para el uso del vial de antígenos para Abrysvo (polvo) y el vial del disolvente
El polvo se debe reconstituir únicamente con el vial del disolvente proporcionado.
- Utilizando una aguja y una jeringa esterilizadas, extraiga todo el contenido del vial con el disolvente e inyecte todo el contenido de la jeringa en el vial con el polvo.
- Agite suavemente el vial con movimientos circulares hasta que el polvo se disuelva por completo. No agite.
- Extraiga 0,5 ml del vial con la vacuna reconstituida.
La vacuna preparada es una solución transparente e incolora. Inspeccione visualmente la vacuna para detectar partículas grandes y cambios de color antes de la administración. No la use si observa partículas grandes o cambios de color.
Eliminación
La eliminación del producto no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ABRYSVO POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 0,5 MLPrincipio activo: respiratory syncytial virus vaccinesFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, 50 µgPrincipio activo: respiratory syncytial virus vaccinesFabricante: Moderna Biotech Spain S.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,5 mlPrincipio activo: ebola vaccinesFabricante: Janssen-Cilag International N.VRequiere receta
Médicos online para ABRYSVO POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ABRYSVO POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes