
MVABEA INJECTABLE SUSPENSION

How to use MVABEA INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Mvabea injectable suspension
Ebola virus vaccine (MVA-BN-Filo [recombinant])
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you or your child are vaccinated, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed to you or your child, do not pass it on to others.
- If you or your child experience any side effects, talk to your doctor, pharmacist, or nurse, even if you think the side effects are not serious. See section 4.
Contents of the pack
- What is Mvabea and what is it used for
- What you need to know before you or your child are given Mvabea
- How Mvabea is given
- Possible side effects
- Storage of Mvabea
- Contents of the pack and other information
1. What is Mvabea and what is it used for
What is Mvabea
Mvabea is a vaccine used for future protection against Ebola virus disease. It is given to people aged 1 year and older who may come into contact with the Ebola virus.
Mvabea is given as the second dose of a 2-dose vaccination schedule that helps protect against Ebola virus disease caused by the Zaire ebolavirus, which is a type of Filovirus. This vaccine will not protect you against other types of Filovirus.
Because Mvabea does not contain the whole Ebola virus, it cannot give you Ebola virus disease.
The 2-dose vaccination schedule consists of:
- a first dose of the Zabdeno vaccine,
- followed about 8 weeks later by a dose of the Mvabea vaccine.
Even after you have received the Zabdeno and Mvabea vaccination schedule, you must still be very careful not to come into contact with the Ebola virus. As with all vaccinations, it is possible that the vaccination schedule may not fully protect all people against Ebola virus disease.
The 2-dose vaccination schedule with Zabdeno and Mvabea should be used in accordance with official recommendations.
What is Ebola
- Ebola is a serious disease caused by a virus. People get Ebola from people or animals that are infected with Ebola or have died from Ebola.
- You can get Ebola through blood and body fluids such as urine, feces, saliva, vomit, sweat, breast milk, semen, and vaginal fluids from people who are infected with the Ebola virus.
- You can also get Ebola through objects that have been in contact with the blood or body fluids of a person or animal with Ebola (such as clothing or objects in direct contact).
- Ebola is not spread through the air, water, or food.
Ebola virus disease usually causes high fever - and this can affect blood clotting, causing severe bleeding (“severe hemorrhagic fever”). This can cause serious illness and, in some cases, death.
- The first signs and symptoms can be fever, tiredness, weakness, or dizziness and muscle pain.
- Later, the signs and symptoms can include bleeding under the skin or in organs of the body such as the liver or kidneys and from the mouth, eyes, or ears. Some people suffer from severe diarrhea or a sudden drop in blood pressure or blood flow to the organs of the body (shock), which can cause serious and permanent damage to these organs, severe confusion (delirium), seizures (epileptic fits), kidney failure, and coma.
Talk to your doctor, pharmacist, or nurse before deciding whether you or your child should be given this vaccine.
How the vaccine works
The 2-dose vaccination schedule with Zabdeno and Mvabea stimulates the body's natural defenses (immune system). The vaccine works by making the body produce its own protection (antibodies) against the virus that causes the Ebola infection. This will help protect you against Ebola virus disease in the future.
2. What you need to know before you or your child are given Mvabea
To make sure that the vaccination schedule is suitable for you or your child, it is important that you tell your doctor, pharmacist, or nurse if any of the following apply to you or your child. If there is anything you do not understand, ask your doctor, pharmacist, or nurse to explain.
Do not have this vaccine if
- you or your child have ever had a severe allergic reaction to any of the active substances or any of the other ingredients listed in section 6.
- you or your child have ever had a severe allergic reaction to chicken or egg or to an antibiotic known as “gentamicin”.
If you are not sure, talk to your doctor, pharmacist, or nurse before you are given the vaccine.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start receiving Mvabea if you or your child:
- have ever had a severe allergic reaction after receiving any other injectable vaccine,
- have ever fainted after an injection,
- have a bleeding problem or bruise easily,
- currently have a fever or infection,
- are taking medicines that weaken the immune system, such as high-dose corticosteroids (e.g., prednisone) or chemotherapy (cancer medicines),
- have a weakened immune system - for example, due to HIV infection or a genetic disorder.
If any of the above apply to you or your child (or if you are unsure), talk to your doctor, pharmacist, or nurse before you are given Mvabea.
If you or your child are at high risk of coming into contact with the Ebola virus, a booster vaccination with Zabdeno may be recommended for you or your child. Talk to your doctor, pharmacist, or nurse if this applies to you or your child.
If you or your child only receive one of the vaccines, Zabdeno or Mvabea, this may offer less protection against Ebola virus disease than if you receive a schedule with both vaccines.
As with all vaccines, it is possible that the 2-dose vaccination schedule with Zabdeno and Mvabea may not fully protect all people against Ebola virus disease and it is not known for how long you will be protected.
- People who have been given the 2-dose vaccination schedule must continue to take precautions to avoid coming into contact with the Ebola virus.
Washing your hands properly is the most effective way to prevent the spread of harmful germs like the Ebola virus. This reduces the number of germs on your hands and their spread from person to person.
The following are described as adequate hand washing methods.
- Use water and soap when your hands are dirty with dirt, blood, or other body fluids. You do not need to use antimicrobial soap for hand washing.
- Use an alcohol-based hand disinfectant when your hands are not dirty. Do not use alcohol-based hand disinfectants when your hands are dirty with dirt, blood, or other body fluids.
While you are in an Ebola-affected area, it is important to avoid the following:
- Contact with blood and body fluids (such as urine, feces, saliva, sweat, vomit, breast milk, semen, and vaginal fluids).
- Items that have been in contact with the blood or body fluids of an infected person (such as clothing, bedding, needles, and medical equipment).
- Funeral or burial rituals that require handling the body of someone who has died from Ebola.
- Contact with bats, monkeys, or apes or with blood, fluids, and raw meat from these animals (bush meat) or meat of unknown origin.
- Contact with the semen of a man who has had Ebola. You should practice safe sex until you know that the virus has disappeared from the semen. Talk to your doctor, pharmacist, or nurse about how long you should practice safe sex.
Children under 1 year of age
Mvabea must not be given to children under 1 year of age.
Other medicines and Mvabea
Tell your doctor or pharmacist if you or your child are using, have recently used, or might use any other medicine or vaccine.
Pregnancy and breastfeeding
If you or your daughter are pregnant or breastfeeding, talk to your doctor or pharmacist before you receive this vaccine. Do this also if you think you or your daughter might be pregnant or plan to become pregnant.
Driving and using machines
Mvabea has no known effects on the ability to drive and use machines.
Mvabea contains sodium
Mvabea contains less than 1 mmol of sodium (23 mg) per 0.5 mL; this is essentially “sodium-free”.
3. How Mvabea is given
Your doctor or nurse will inject you in a muscle (intramuscular injection) in the upper arm or thigh.
Mvabea must not be injected into a blood vessel.
The 2-dose vaccination schedule consists of:
- a dose of the Zabdeno vaccine,
- followed about 8 weeks later by a dose of the Mvabea vaccine.
Your doctor will tell you the date of the second vaccination.
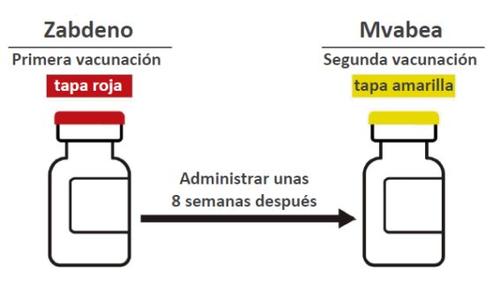
How much vaccine will you or your child receive Primovaccination
- First vaccination with Zabdeno - vial with red cap (0.5 mL).
- Second vaccination with Mvabea - vial with yellow cap (0.5 mL), given about 8 weeks after the first vaccination with Zabdeno.
Booster vaccination with Zabdeno (an additional dose of Zabdeno to increase or renew the effect of the previously received 2-dose vaccination schedule with Zabdeno and Mvabea)
- Booster vaccination is recommended for you or your child if you are at high risk of coming into contact with the Ebola virus and have completed the 2-dose vaccination schedule more than 4 months ago.
- Ask your doctor if you or your child should receive a booster vaccination.
During and after the injection of the vaccine, the doctor will observe you or your child for 15 minutes, or longer if necessary, in case of a severe allergic reaction.
At the end of this leaflet, there are instructions for preparing the vaccine- for healthcare professionals.
If you or your child accidentally receive an injection of Zabdeno or Mvabea
- If you or your child are accidentally given Mvabea as the first vaccination - you will receive Zabdeno as the second vaccination about 8 weeks later.
- If you or your child are accidentally given Zabdeno as the first and second vaccinations - you will receive Mvabea about 8 weeks after the second vaccination with Zabdeno.
- If you or your child are accidentally given Mvabea as the first and second vaccinations - you will receive Zabdeno about 8 weeks after the second vaccination with Mvabea.
- If you or your child have not been given Mvabea about 8 weeks after vaccination with Zabdeno - talk to your doctor, pharmacist, or nurse about receiving the second vaccination with Mvabea.
If you miss a vaccination appointment with Zabdeno or Mvabea
- If you miss an appointment, inform your doctor and make a new appointment.
- If you forget a scheduled injection, you may not be fully protected against the Ebola virus.
- If you have any other questions about the use of this vaccine, ask your doctor.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them. Most side effects happened within 7 days of receiving the injection.
The following side effects may occur in adults.
Very common(may affect more than 1 in 10 people)
- pain, warmth, or swelling at the injection site
- feeling very tired
- muscle pain
- joint pain
Common(may affect up to 1 in 10 people)
- vomiting
- itching at the injection site
Uncommon(may affect up to 1 in 100 people)
- redness and hardening of the skin at the injection site
- generalized itching
The following side effects may occur in children and adolescents from 1 to 17 years of age.
Very common(may affect more than 1 in 10 people)
- pain at the injection site
- feeling very tired
Common(may affect up to 1 in 10 people)
- swelling, itching, or redness at the injection site
- fever
- chills
- muscle pain
- joint pain
Most of these side effects are mild or moderate and do not last long.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if you think the side effects are not serious. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Mvabea
Keep this vaccine out of the sight and reach of children.
Information on storage, expiry, and use and disposal is described in the section for healthcare professionals at the end of the leaflet.
Your doctor or pharmacist is responsible for storing this vaccine and disposing of any unused product correctly.
6. Container Contents and Additional Information
Mvabea Composition
A dose (0.5 mL) contains:
- The active ingredient is the modified vaccinia Ankara virus Bavarian Nordic* that encodes:
- the glycoprotein (GP) of the Mayinga variant of Zaire ebolavirus
- the GP of the Gulu variant of Sudan ebolavirus
- the nucleoprotein of Tai Forest ebolavirus
- the GP of the Musoke variant of Marburg marburgvirus
Not less than 0.7 x 10^8 infectious units
- Produced in chicken embryo fibroblasts and using recombinant DNA technology.
This product contains genetically modified organisms (GMOs).
This vaccine contains minimal residues of gentamicin (see section 2).
- The other components (excipients) are sodium chloride, trometamol, water for injectable preparations, and hydrochloric acid (for pH adjustment).
Appearance of Mvabea and Container Contents
Mvabea is a suspension in a single-dose glass vial (0.5 mL) with a rubber stopper and yellow cap.
Clear to cloudy yellow suspension.
Mvabea is presented in a container containing 20 single-dose vials.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
Netherlands
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
България „Джонсън & Джонсън“ ЕООД Тел.: +359 2 489 94 00 | Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Česká republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: +49 2137 955 955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλάδα Janssen-Cilag Φαρμακευτική Α.Ε.Β.Ε. Τηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
Κύπρος Βαρνάβας Χατζηπαναγής Λτδ Τηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom(Northern Ireland) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Date of Last Revision of this Prospectus: <{MM/AAAA}> <{month AAAA}>.
This vaccine has been authorized under "exceptional circumstances". This authorization means that, for scientific reasons, it has not been possible to obtain complete information on this medicinal product. The European Medicines Agency will review the new information on this vaccine once a year and this prospectus will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
The prospectus can be found on the European Medicines Agency website in all languages of the European Union/European Economic Area.
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
- As with all injectable vaccines, appropriate medical treatment and supervision should always be readily available in case of anaphylactic reaction following administration of Mvabea. Individuals should be observed by a healthcare professional for at least 15 minutes after vaccination.
- Mvabea should not be mixed with other medicinal products in the same syringe.
- Mvabea should not be administered by intravascular injection under any circumstances.
- Vaccination should be performed by intramuscular (IM) injection, preferably in the deltoid region of the arm or in the thigh.
- A syncopal reaction (fainting) may occur after, or even before, any vaccination as a psychogenic response to the needle injection. Procedures should be in place to avoid injury from fainting and to manage syncopal reactions.
Administration and Handling Instructions
Mvabea is a clear to cloudy yellow suspension. Before administration, a visual inspection of the vaccine should be performed to rule out the presence of particles and color changes. The vial should be inspected visually before administration for cracks or anomalies such as signs of tampering. If any of these signs are present, do not administer the vaccine.
Once removed from the freezer and thawed, use immediately or store in the refrigerator at a temperature of 2°C to 8°C (see section 6.4). Once removed from the refrigerator for administration, it should be used immediately.
Gently mix the contents of the vial by rotating it for 10 seconds. Do not shake. Use a sterile needle and a sterile syringe to withdraw the entire contents of the vial for administration.
Use a different sterile needle and syringe for each person. It is not necessary to change the needle used to withdraw the vaccine from the vial before injecting it into the individual, unless the needle has been damaged or contaminated. Any remaining contents in the vial should be discarded.
Disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local regulations. Any spills should be disinfected with agents that have virucidal activity against the vaccinia virus.
Storage Information
Do not use this vaccine after the expiration date stated on the carton after CAD. The expiration date is the last day of the month indicated.
Transport frozen between -25°C and -15°C. Once received, the product can be stored as follows:
Store in a freezer between -85°C and -55°C in the case of storage by the distributor. The expiration date when stored between -85°C and -55°C is printed on the vial and on the outer carton after CAD. The distributor or the final user may also store the vaccine in a freezer between -25°C and -15°C for a single period of up to 7 months. When removed from the freezer at a temperature between -85°C and -55°C, the distributor or the final user should record the new expiration date on the outer carton and the vaccine should be used or discarded at the end of the 7 months. This new expiration date should not exceed the original expiration date (CAD). The original expiration date should be made illegible.
The distributor or the final user may also store the vaccine in a refrigerator between 2°C and 8°C for a single period of up to 1 month. When the product is transferred to a storage temperature between 2°C and 8°C, the distributor or the final user should record the elimination date on the outer carton and the vaccine should be used or discarded at the end of the 1-month period. This elimination date should not exceed the original expiration date (CAD) or the new expiration date assigned for storage conditions between -25°C and -15°C. The original expiration date and/or the new expiration date assigned for storage between -25°C and -15°C should be made illegible.
Once thawed, the vaccine cannot be re-frozen.
The vial should be kept in the original packaging to protect it from light and for tracking of the expiration date or elimination date depending on the different storage conditions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MVABEA INJECTABLE SUSPENSIONDosage form: INJECTABLE, 0.50 mlActive substance: ebola vaccinesManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 0.5 mLActive substance: respiratory syncytial virus vaccinesManufacturer: Glaxosmithkline BiologicalsPrescription required
Online doctors for MVABEA INJECTABLE SUSPENSION
Discuss questions about MVABEA INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















