
ABILIFY MAINTENA 400 MG POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA


Cómo usar ABILIFY MAINTENA 400 MG POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Abilify Maintena 300 mg polvo y disolvente para suspensión de liberación prolongada inyectable
Abilify Maintena 400 mg polvo y disolvente para suspensión de liberación prolongada inyectable
aripiprazol
Lea todo el prospecto detenidamente antes de recibir este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Abilify Maintena y para qué se utiliza
- Qué necesita saber antes de que se le administre Abilify Maintena
- Como se administra Abilify Maintena
- Posibles efectos adversos
- Conservación de Abilify Maintena
- Contenido del envase e información adicional
1. Qué es Abilify Maintena y para qué se utiliza
Abilify Maintena contiene el principio activo aripiprazol y pertenece a un grupo de medicamentos llamados antipsicóticos. Se utiliza para tratar la esquizofrenia – una enfermedad con síntomas como escuchar, ver o sentir cosas que no existen, desconfianza, creencias equivocadas, lenguaje incoherente y monotonía emocional y del comportamiento. Las personas con esta enfermedad también pueden sentirse deprimidas, culpables, inquietas o tensas.
Abilify Maintena está indicado para pacientes adultos con esquizofrenia que están suficientemente estabilizados durante el tratamiento con aripiprazol oral.
2. Qué necesita saber antes de que se le administre Abilify Maintena
No use Abilify Maintena:
- si es alérgico al aripiprazol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Hable con su médico o enfermero antes de que le administren Abilify Maintena.
Se han comunicado casos de pacientes que experimentan pensamientos y comportamientos suicidas durante el tratamiento con aripiprazol. Informe a su médico inmediatamente si tiene pensamientos o sentimientos de dañarse a sí mismo.
Antes del tratamiento con Abilify Maintena, dígale a su médico si sufre de
- un estado de agitación aguda o un estado intensamente psicótico.
- problemas cardíacos o antecedentes de accidente cerebrovascular, especialmente si sabe que en usted concurren otros factores de riesgo para el accidente cerebrovascular.
- niveles altos de azúcar en la sangre (caracterizado por síntomas como sed excesiva, aumento de la cantidad de orina, aumento del apetito y sensación de debilidad) o antecedentes familiares de diabetes.
- convulsiones, ya que su médico puede querer controlarlo más de cerca.
- movimientos musculares involuntarios e irregulares, especialmente en la cara.
- presenta una combinación de fiebre, sudoración, respiración acelerada, rigidez muscular y somnolencia o adormecimiento (pueden ser signos de síndrome neuroléptico maligno).
- demencia (pérdida de memoria y de otras capacidades mentales) especialmente si es mayor.
- enfermedades cardiovasculares (enfermedades del corazón y la circulación), antecedentes familiares de enfermedad cardiovascular, ictus o mini ictus, presión sanguínea anormal.
- latidos cardíacos irregulares o si alguien más en su familia tiene antecedente de latidos cardíacos irregulares (incluida la denominada prolongación del intervalo QT observada con la monitorización por ECG).
- coágulos sanguíneos o antecedentes familiares de coágulos sanguíneos ya que los antipsicóticos han sido asociados con la formación de coágulos sanguíneos.
- tiene alguna dificultad para tragar.
- antecedentes de adicción al juego.
- problemas de hígado (hepáticos) graves.
Si nota que está ganando peso, desarrolla movimientos inusuales, siente somnolencia que interfiere en sus actividades diarias normales, cualquier dificultad para tragar o presenta síntomas de alergia, hable con su médico inmediatamente.
Informe a su médico si usted, su familia o cuidador notan que está desarrollando impulsos o ansias de comportarse de forma inusual en usted y que no se puede resistir al impulso, instinto o tentación de llevar a cabo ciertas actividades que pueden dañarle a usted o a otros. Esto se denomina trastorno del control de los impulsos y puede incluir comportamientos como adicción al juego, ingesta o gasto excesivo, apetito sexual anormalmente alto o preocupación por un aumento de los pensamientos y sentimientos sexuales.
Su médico puede considerar ajustar o interrumpir la dosis.
El aripiprazol puede causar somnolencia, caída de la tensión arterial al levantarse, mareos y cambios en la capacidad para moverse y mantener el equilibrio, lo que podría provocar caídas. Se debe tener precaución, especialmente si usted es un paciente anciano o padece algo de debilidad.
Niños y adolescentes
No utilice este medicamento en niños y adolescentes menores de 18 años. Se desconoce si es seguro y efectivo en estos pacientes.
Uso de Abilify Maintena con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tomar cualquier otro medicamento.
Medicamentos para bajar la presión sanguínea: Abilify Maintena puede aumentar el efecto de medicamentos utilizados para bajar la presión sanguínea. Asegúrese de informar a su médico si usted toma medicamentos para controlar la presión sanguínea.
Si está usando Abilify Maintena con algún otro medicamento, puede significar que su médico deba cambiar su dosis de Abilify Maintena o la de los otros medicamentos. Es especialmente importante que mencione a su médico si está tomando:
- medicamentos para corregir el ritmo cardíaco (como quinidina, amiodarona, flecainida)
- antidepresivos o medicamentos a base de plantas utilizados para el tratamiento de la depresión y la ansiedad (como fluoxetina, paroxetina, hierba de San Juan)
- medicamentos para tratar infecciones por hongos (antifúngicos) (como ketoconazol, itraconazol)
- ciertos medicamentos para tratar la infección por VIH (como efavirenz, nevirapina e inhibidores de la proteasa como, por ejemplo, indinavir, ritonavir)
- anticonvulsivantes utilizados para tratar la epilepsia (como carbamazepina, fenitoína, fenobarbital)
- ciertos antibióticos utilizados para tratar la tuberculosis (rifabutina, rifampicina)
- medicamentos que se sabe que prolongan el intervalo QT.
Estos medicamentos pueden aumentar el riesgo de la aparición de efectos adversos o reducir el efecto de Abilify Maintena; si usted observa cualquier síntoma poco común al tomar cualquiera de estos medicamentos al mismo tiempo que Abilify Maintena, debe comunicárselo a su médico.
Los medicamentos que aumentan los niveles de serotonina se emplean generalmente en enfermedades que incluyen depresión, trastorno de ansiedad generalizada, trastorno obsesivo compulsivo (TOC) y fobia social así como migraña y dolor:
- triptanes, tramadol y triptófano utilizados para enfermedades como la depresión, el trastorno de ansiedad generalizada, el trastorno obsesivo compulsivo (TOC) y la fobia social así como la migraña y el dolor
- ISRS (como paroxetina y fluoxetina) utilizados para la depresión, el TOC, el pánico y la ansiedad
- otros antidepresivos (como venlafaxina y triptófano) utilizados en la depresión grave
- antidepresivos tricíclicos (como clomipramina y amitriptilina) utilizados en enfermedades depresivas
- hierba de San Juan (Hypericum perforatum) utilizada en medicamentos a base de plantas para la depresión leve
- analgésicos (como tramadol y petidina) utilizados para aliviar el dolor
- triptanos (como sumatriptán y zolmitripitán) utilizados para tratar la migraña.
Estos medicamentos pueden aumentar el riesgo de aparición de efectos adversos; si usted observa cualquier síntoma poco común al tomar cualquiera de estos medicamentos al mismo tiempo que Abilify Maintena, debe comunicárselo a su médico.
Uso de Abilify Maintena con alcohol
Se debe evitar el consumo de alcohol.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe usar Abilify Maintena si está embarazadaa no ser que haya hablado esto con su médico. Asegúrese de informar a su médico inmediatamente si está embarazada, cree que puede estar embarazada o si está planeando quedarse embarazada.
Los siguientes síntomas pueden ocurrir en bebés recién nacidos, de madres que han usado Abilify Maintena en el último trimestre de embarazo (últimos tres meses del embarazo): temblor, rigidez y/o debilidad muscular, somnolencia, agitación, problemas respiratorios y dificultad en la alimentación. Si su bebé desarrolla cualquiera de estos síntomas debe contactar con su médico.
Si está usando Abilify Maintena, su médico discutirá con usted sobre si debe dar el pecho a su bebé considerando el beneficio para usted de su tratamiento y el beneficio para su bebé de darle el pecho. Si está siendo tratada con Abilify Maintena no debe dar el pecho. Hable con su médico sobre el mejor modo de alimentar a su bebé si está usando Abilify Maintena.
Conducción y uso de máquinas
Durante el tratamiento con este medicamento pueden aparecer mareos y problemas de visión (ver sección 4). Esto debe tenerse en cuenta cuando se requiera una atención máxima, por ejemplo, cuando conduzca o maneje maquinaria.
Abilify Maintena contiene sodio
Abilify Maintena contiene menos de 1 mmol de sodio (23 mg) por dosis. Esto es, esencialmente «exento de sodio».
3. Cómo se administra Abilify Maintena
Abilify Maintena se presenta en polvo, que su médico o enfermera preparan en una suspensión.
Su médico decidirá cuál es la dosis de Abilify Maintena más adecuada para usted. La dosis inicial recomendada es de 400 mg, a no ser que su médico decida administrarle una dosis inicial más baja o de seguimiento (300 mg, 200 mg o 160 mg).
Hay dos maneras de empezar el tratamiento con Abilify Maintena, su médico decidirá qué manera es la adecuada para usted.
- Si se le administra una inyección de Abilify Maintena en su primer día, el tratamiento con aripiprazol por vía oral se continúa durante 14 días después de la primera inyección.
- Si se le administran dos inyecciones de Abilify Maintena en su primer día, también tomará un comprimido de aripiprazol por vía oral en esta visita.
Después de eso, el tratamiento se administra con inyecciones de Abilify Maintena a menos que su médico le indique lo contrario.
Su médico se lo administrará como una inyección única en el glúteo o el deltoides (nalga u hombro) cada mes. Puede que sienta un poco de dolor durante la inyección. Su médico alternará las inyecciones entre el lado derecho y el izquierdo. Las inyecciones no se administrarán por vía intravenosa.
Si se le administra más Abilify Maintena del que se debe
Este medicamento se le administrará bajo supervisión médica, por lo tanto, es poco probable que se le administre demasiado. Si es visto por más de un médico, asegúrese de decirles que está usando Abilify Maintena.
Los pacientes a los que se les ha administrado demasiado aripiprazol han experimentado los siguientes síntomas:
- latidos rápidos del corazón, agitación/agresividad, problemas con el lenguaje.
- movimientos inusuales (especialmente de la cara o la lengua) y nivel de conciencia disminuido
Otros síntomas pueden incluir:
- confusión aguda, convulsiones (epilepsia), coma, una combinación de fiebre, respiración acelerada, sudoración,
- rigidez muscular y somnolencia, respiración más lenta, ahogo, presión sanguínea alta o baja, ritmos anómalos del corazón.
Contacte con su médico u hospital más cercano inmediatamente si experimenta cualquiera de los síntomas anteriores.
Si no recibió su dosis de Abilify Maintena
Es importante no olvidarse de su dosis programada. Debe recibir una inyección cada mes pero no antes de que hayan pasado 26 días desde la última inyección. Si olvida una inyección, debe contactar con su médico para programar la siguiente inyección lo más pronto posible.
Si se suspende la administración de Abilify Maintena
No interrumpa su tratamiento sólo porque se sienta mejor. Es importante que siga recibiendo Abilify Maintena durante el tiempo que su médico le haya indicado.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico inmediatamente si presenta cualquiera de los siguientes efectos adversos graves:
- una combinación de cualquiera de estos síntomas: somnolencia excesiva, mareos, confusión, desorientación, dificultad para hablar, dificultad para caminar, rigidez muscular o temblor, fiebre, debilidad, irritabilidad, agresividad, ansiedad, elevación de la presión sanguínea o convulsiones que pueden conducir a pérdida del conocimiento.
- movimientos inusuales principalmente de la cara o la lengua, ya que su médico puede querer bajar la dosis.
- si tiene síntomas como hinchazón, dolor y enrojecimiento en la pierna puede ser que tenga un coágulo sanguíneo, que puede desplazarse por los vasos sanguíneos a los pulmones causando dolor en el pecho y dificultad para respirar. Si nota cualquiera de estos síntomas acuda inmediatamente a su médico.
- una combinación de fiebre, respiración más rápida, sudoración, rigidez muscular y somnolencia o letargo ya que esto puede ser un signo de una enfermedad llamada síndrome neuroléptico maligno (SNM).
- más sed de lo habitual, necesita orinar más de lo habitual, con mucho apetito, se siente débil o cansado, siente ganas de vomitar, se siente confundido o su aliento huele a frutas ya que esto puede ser un signo de diabetes.
Los efectos adversos incluidos a continuación también pueden ocurrir después de la administración de Abilify Maintena.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas):
- aumento de peso
- diabetes mellitus
- pérdida de peso
- sentirse inquieto
- sentirse ansioso
- incapaz de quedarse quieto, dificultad para mantenerse sentado
- problemas para dormir (insomnio)
- resistencia espasmódica a movimientos pasivos como tensar y relajar los músculos, tono muscular aumentado en forma anómala, movimientos lentos del cuerpo
- acatisia (una sensación incómoda de inquietud interna y una necesidad imperiosa de moverse constantemente)
- crisis convulsivas o temblores
- tics incontrolables, sacudidas o movimientos de retorcimiento
- cambios en el nivel de lucidez, adormecimiento
- somnolencia
- mareo
- dolor de cabeza
- boca seca
- rigidez muscular
- incapacidad para tener o mantener una erección durante las relaciones sexuales
- dolor en el sitio de la inyección, endurecimiento de la piel en el sitio de la inyección
- debilidad, pérdida de fuerza o cansancio extremo
- durante los análisis de sangre, su médico puede encontrar cantidades elevadas de creatina fosfocinasa en la sangre (enzima importante para la función muscular)
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- niveles bajos de un cierto tipo de glóbulos blancos (neutropenia), hemoglobina baja o bajo recuento de glóbulos rojos, bajo recuento de plaquetas en la sangre
- reacción alérgica (hipersensibilidad)
- aumento o disminución de los niveles sanguíneos de la hormona prolactina
- aumento de azúcar en la sangre
- aumento de grasas sanguíneas como colesterol, triglicéridos elevados y también niveles bajos de colesterol y niveles bajos de triglicéridos
- aumento de los niveles de insulina, una hormona que regula los niveles del azúcar en la sangre
- aumento o disminución del apetito
- pensamientos de suicidio
- trastorno mental caracterizado por percepción defectuosa o pérdida de la realidad
- alucinaciones
- delirio
- interés sexual aumentado
- reacción de pánico
- depresión
- inestabilidad emocional
- estado de indiferencia con falta de emoción, sentimientos de malestar emocional y mental
- trastorno del sueño
- rechinar los dientes o apretar la mandíbula
- menor interés sexual (la libido disminuye)
- estado de ánimo alterado
- problemas musculares
- movimientos musculares que no puede controlar, como muecas, chasquear los labios o movimientos de la lengua. Generalmente afectan a la cara y la boca primero pero pueden afectar a otras partes del cuerpo. Estos pueden ser signos de un trastorno llamado “discinesia tardía”.
- parkinsonismo: afección con muchos y variados síntomas que incluyen movimientos lentos o disminuidos, lentitud de pensamiento, tirones al doblar las extremidades (rigidez en rueda dentada), arrastrar los pies, pasos apresurados, temblores, expresión facial escasa o ausente, rigidez muscular, babeo
- problemas de movimiento
- resistencia extrema y piernas inquietas
- distorsión de los sentidos del gusto y del olfato
- fijación de los globos oculares en una posición
- visión borrosa
- dolor de ojos
- visión doble
- fotosensibilidad ocular
- latido anómalo del corazón, velocidad del latido rápida o lenta, conducción eléctrica del corazón anómala, electrocardiograma anómalo (ECG)
- presión arterial alta
- mareo al ponerse de pie después de haber estado acostado o sentado debido a una bajada en la presión arterial
- tos
- hipo
- enfermedad por reflujo gastroesofágico. Cantidad excesiva de jugo gástrico que retrocede (reflujos) hacia el interior del esófago (garganta o el tubo que va de la boca al estómago a través del cual pasan los alimentos), causando acidez estomacal y posiblemente dañando el esófago
- acidez estomacal
- vómitos
- diarrea
- náuseas
- dolor de estómago
- malestar estomacal
- estreñimiento
- movimientos intestinales frecuentes
- babeo, más saliva en la boca de lo normal
- caída anormal del cabello
- acné, enfermedad de la piel donde la nariz y las mejillas están inusualmente enrojecidas, eczema, endurecimiento de la piel
- rigidez muscular, espasmos musculares, tics musculares, tensión muscular, dolor muscular (mialgia), dolor en las extremidades
- dolor de las articulaciones (artralgia), dolor de espalda, disminución de la movilidad de las articulaciones, cuello rígido, apertura de la boca limitada
- cálculos renales o azúcar (glucosa) en la orina
- secreción espontánea de leche por las mamas (galactorrea)
- aumento del tamaño de las mamas en los hombres, mamas dolorosas, sequedad vaginal
- fiebre
- pérdida de fuerza
- alteración de la marcha
- molestias en el pecho
- reacciones en el sitio de inyección como enrojecimiento, hinchazón, malestar y picor en el sitio de la inyección
- sed
- lentitud
- las pruebas de función hepática pueden mostrar resultados anómalos
- durante las pruebas su médico puede encontrar:
- cantidades elevadas de enzimas hepáticas
- cantidades elevadas de alanina aminotransferasa
- cantidades elevadas de γ-glutamil transferasa
- cantidades elevadas de bilirrubina en su sangre
- cantidades elevadas de aspartato aminotransferasa
- cantidades elevadas o reducidas de glucosa en sangre
- cantidades elevadas de hemoglobina glucosilada
- cantidades reducidas de colesterol en la sangre
- cantidades reducidas de triglicéridos en la sangre
- un mayor perímetro de la cintura
Se han notificado los siguientes efectos adversos desde la comercialización de aripiprazol oral, pero se desconoce la frecuencia con que se producen (la frecuencia no puede estimarse a partir de los datos disponibles):
- niveles bajos de glóbulos blancos
- reacción alérgica (p. ej., hinchazón de la boca, lengua, rostro y garganta, picor, urticaria), sarpullido
- latido cardíaco inusual, muerte súbita inexplicada, ataque al corazón
- cetoacidosis diabética (cetonas en la sangre y la orina) o coma
- pérdida del apetito (anorexia), dificultad para tragar
- bajo nivel de sodio en la sangre
- intento de suicidio y suicidio consumado
- incapacidad de resistir el impulso, instinto o tentación de realizar una acción que puede ser dañina para usted o para otros, pudiendo incluir:
- fuerte impulso de jugar excesivamente a pesar de las serias consecuencias personales o familiares
- interés sexual alterado o aumentado y comportamiento preocupante para usted o para otros, por ejemplo, aumento del apetito sexual
- compra excesiva incontrolable
- atracón (ingesta de grandes cantidades de comida en un corto periodo de tiempo) o ingesta compulsiva (ingesta de más comida de lo normal y más de la necesaria para satisfacer el hambre)
- tendencia a deambular
Informe a su médico si presenta alguno de estos comportamientos; él le explicará la manera de manejar o reducir los síntomas.
- nerviosismo
- agresividad
- síndrome neuroléptico maligno (un síndrome con síntomas como fiebre, rigidez muscular, respiración acelerada, sudoración, disminución de la conciencia y cambios repentinos en la presión arterial y la frecuencia cardíaca)
- convulsiones (ataques)
- síndrome serotoninérgico (una reacción que puede causar sensación de intensa felicidad, somnolencia, torpeza, inquietud, sensación de estar bebido, fiebre, sudoración, rigidez muscular)
- trastornos del habla
- problemas cardíacos, incluyendo taquicardia ventricular helicoidal, paro cardíaco, irregularidades en el ritmo cardíaco que pueden deberse a impulsos nerviosos anormales en el corazón, lecturas anormales durante el examen cardíaco (ECG), prolongación del intervalo QT
- desmayo
- síntomas relacionados con coágulos de sangre en las venas, especialmente en las piernas (los síntomas incluyen hinchazón, dolor y enrojecimiento en la pierna), que pueden viajar a través de los vasos sanguíneos hasta los pulmones, causando dolor en el tórax y dificultad para respirar
- espasmo de los músculos alrededor de la glotis
- aspiración accidental de alimento con riesgo de neumonía (infección de los pulmones)
- inflamación del páncreas
- dificultad para tragar
- insuficiencia hepática
- ictericia (color amarillo de la piel y la parte blanca de los ojos)
- inflamación del hígado
- sarpullido
- fotosensibilidad cutánea
- sudoración excesiva
- reacciones alérgicas graves, como la reacción a fármaco con eosinofilia y síntomas sistémicos (síndrome DRESS). El síndrome DRESS aparece inicialmente como síntomas pseudogripales con erupción cutánea en el rostro y, más adelante, con erupción cutánea prolongada, temperatura alta, ganglios linfáticos agrandados, aumento de las concentraciones de enzimas hepáticas observado en los análisis de sangre y aumento de un tipo de glóbulos blancos (eosinofilia)
- debilidad muscular, sensibilidad o dolor y particularmente, si al mismo tiempo se siente mal, tiene una temperatura alta o tiene orina oscura. Pueden ser causados por un metabolismo muscular anormal que es potencialmente mortal y provocar problemas renales (una afección llamada rabdomiolisis)
- dificultad para orinar
- pérdida involuntaria de orina (incontinencia)
- síntomas de abstinencia en recién nacidos
- erección prolongada y/o dolorosa
- dificultad para controlar la temperatura corporal central o sobrecalentamiento
- dolor en el pecho
- hinchazón de las manos, tobillos o pies
- durante las pruebas su médico puede hallar:
- cantidades elevadas de fosfatasa alcalina
- resultados fluctuantes durante las pruebas para medir la glucosa en la sangre
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Abilify Maintena
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y el vial. La fecha de caducidad es el último día del mes que se indica.
No congelar.
La suspensión reconstituida debe utilizarse inmediatamente pero puede ser conservada en el vial durante 4 horas a una temperatura inferior a 25 °C. No conserve la suspensión reconstituida en la jeringa.
6. Contenido del envase e información adicional
Composición de Abilify Maintena
- El principio activo es aripiprazol.
Cada vial contiene 300 mg de aripiprazol.
Después de la reconstitución, cada ml de suspensión contiene 200 mg de aripiprazol. Cada vial contiene 400 mg de aripiprazol.
Después de la reconstitución, cada ml de suspensión contiene 200 mg de aripiprazol.
- Los demás componentes son
Polvo
Carmelosa sódica, manitol, fosfato deshidrogenado de sodio monohidrato, hidróxido de sodio
Disolvente
Agua para preparaciones inyectables
Aspecto de Abilify Maintena y contenido del envase
Abilify Maintena es un polvo y disolvente para la preparación de una suspensión inyectable de liberación prolongada.
Abilify Maintena es un polvo blanco o blanquecino que se presenta en un vial de vidrio transparente. Su médico o enfermero/a preparará una suspensión de Abilify Maintena que se le administrará en una inyección. Para ello utilizará el vial de disolvente que viene en el estuche, solución transparente en un vial de vidrio transparente.
Envase individual
Cada envase individual contiene un vial con polvo, un vial de 2 ml con disolvente, una jeringa de 3 ml con luer-lock con una aguja hipodérmica de seguridad ya colocada, de 38 mm, calibre 21, con un dispositivo de protección de la aguja, una jeringa desechable de 3 ml con punta luer-lock, un adaptador de vial, y tres agujas hipodérmicas de seguridad: una de 25 mm y calibre 23, una de 38 mm y calibre 22, y una de 51 mm y calibre 21.
Envase múltiple
Lote de 3 envases individuales.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercializaciónOtsuka Pharmaceutical Netherlands B.V. Herikerbergweg 292
1101 CT, Amsterdam
Países Bajos
Responsable de la fabricación
- Lundbeck A/S Ottiliavej 9, 2500 Valby
Dinamarca
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Lundbeck S.A./N.V. Tél/Tel: +32 2 535 79 79 | Lietuva
Tel: +45 36301311 |
???????? Lundbeck Export A/S Representative Office Tel: +359 2 962 4696 | Luxembourg/Luxemburg Lundbeck S.A. Tél: +32 2 535 79 79 |
Ceská republika Lundbeck Ceská republika s.r.o. Tel: +420 225 275 600 | Magyarország Lundbeck Hungaria Kft. Tel: +36 1 4369980 |
Danmark Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 | Malta
Tel: +45 36301311 |
Deutschland Otsuka Pharma GmbH Tel: +49 69 1700860 | Nederland Lundbeck B.V. Tel: +31 20 697 1901 |
Eesti
Tel: +45 36301311 | Norge Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 |
Ελλ?δα Lundbeck Hellas S.A. Τηλ: +30 210 610 5036 | Österreich Lundbeck Austria GmbH Tel: +43 1 266 91 08 |
España Otsuka Pharmaceutical S.A. Tel: +34 93 208 10 20 | Polska Lundbeck Poland Sp. z o. o. Tel.: +48 22 626 93 0 |
France Otsuka Pharmaceutical France SAS Tél: +33 (0) 1 47 08 00 00 | Portugal Lundbeck Portugal Lda Tel: +351 21 00 45 900 |
Hrvatska Lundbeck Croatia d.o.o. Tel.: +385 1 644 82 63 | România Lundbeck Export A/S Reprezentanta din Romania Tel: +40 21319 88 26 |
Ireland Lundbeck (Ireland) Limited Tel: +353 1 468 980 | Slovenija Lundbeck Pharma d.o.o. Tel.: +386 2 229 4500 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Lundbeck Slovensko s.r.o. Tel: +421 2 5341 42 18 |
Italia Otsuka Pharmaceutical Italy S.r.l Tel: +39 02 00 63 27 10 | Suomi/Finland Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 |
Κ?προς Lundbeck Hellas A.E Τηλ.: +357 22490305 | Sverige Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 |
Latvija
Tel: +45 36301311 | United Kingdom Otsuka Pharmaceuticals (UK) Ltd. Tel: +44 203 747 5300 |
Fecha de la última revisión de este prospecto: {MM/AAAA}.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu.
-----------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
INSTRUCCIONES PARA PROFESIONALES SANITARIOS
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
Abilify Maintena 300 mg polvo y disolvente para suspensión de liberación prolongada inyectable
Abilify Maintena 400 mg polvo y disolvente para suspensión de liberación prolongada inyectable
Aripiprazol
Paso 1: Preparación antes de reconstituir el polvo
Saque todos los componentes del envase y confirme que están todos los que se indican en la siguiente lista:
- Prospecto de Abilify Maintena e instrucciones para profesionales sanitarios
- Vial con polvo.
- Vial con 2 ml de disolvente.
Importante:el vial con el disolvente contiene una cantidad adicional de disolvente.
- Una jeringa luer-lock de 3 ml con aguja hipodérmica de seguridad de 38 mm calibre 21, ya colocada, con un dispositivo de protección para la aguja.
- Una jeringa desechable con punta luer-lock de 3 ml.
- Un adaptador del vial.
- Una aguja hipodérmica de seguridad de 25 mm calibre 23 con dispositivo de protección para la aguja.
- Una aguja hipodérmica de seguridad de 38 mm calibre 22 con dispositivo de protección para la aguja.
- Una aguja hipodérmica de seguridad de 51 mm calibre 21 con dispositivo de protección para la aguja.
- Instrucciones para la aguja y la jeringa
Paso 2: Reconstitución del polvo
- Quite la tapa del vial con polvo y la del vial con disolvente y limpie el tapón de ambos viales con un hisopo estéril impregnado en alcohol.
- Utilizando la jeringa con la aguja ya colocada, extraiga el volumen de disolvente predeterminado del vial correspondiente, volcando el disolvente hacia la jeringa.
Vial de 300 mg:
Añada 1,5 ml de disolvente para reconstituir el polvo.
Vial de 400 mg:
Añada 1,9 ml de disolvente para reconstituir el polvo.
Quedará una pequeña cantidad de disolvente residual en el vial después de la extracción. Cualquier exceso debe ser eliminado.

Agua
- Inyecte lentamente el disolvente en el vial que contiene el polvo.
- Saque el aire para igualar la presión en el vial tirando del émbolo levemente hacia atrás.

- Posteriormente, saque la jeringa del vial.
Proceda a encajar el dispositivo de seguridad de la aguja utilizando la técnica de una mano.
Presione suavemente la funda contra una superficie plana hasta que la aguja quede encajada firmemente en la funda de protección de la aguja.
Compruebe visualmente que la aguja ha encajado perfectamente en la funda de protección de la aguja y deseche la jeringa.

- Agite el vial vigorosamente durante 30 segundos como mínimo hasta que la suspensión se vea uniforme.
- Inspeccione visualmente la suspensión reconstituida por si hubiera partículas y decoloración antes de la administración. El medicamento reconstituido es una suspensión líquida de color blanco o blanquecino. No utilice la suspensión reconstituida si contiene partículas o presenta decoloración.
- Si no se administra la inyección inmediatamente después de la reconstitución, puede mantener el vial hasta 4 horas a una temperatura inferior a 25 °C y agitarlo vigorosamente, durante al menos 60 segundos, para garantizar la resuspensión antes de inyectarla.
- No conserve la suspensión reconstituida en la jeringa.
Paso 3: Preparación antes de la inyección
- Retire la cubierta del envase protector del adaptador del vial, pero no extraiga el adaptador del vial del envase protector.
- Sujete el envase protector del adaptador del vial, y fije la jeringa luer-lock insertándola en el adaptador del vial y girándola a continuación.

- Emplee la jeringa luer-lock para extraer el adaptador del vial del envase protector y deseche el envase protector del adaptador del vial. No toque la punta del adaptador del vial en ningún momento.

- Determine el volumen recomendado para la inyección.
Abilify Maintena vial de 300 mg | |
Dosis | Volumen a inyectar |
--- | --- |
300 mg | 1,5 ml |
200 mg | 1,0 ml |
160 mg | 0,8 ml |
Abilify Maintena vial de 400 mg | |
Dosis | Volumen a inyectar |
400 mg | 2,0 ml |
300 mg | 1,5 ml |
200 mg | 1,0 ml |
160 mg | 0,8 ml |
- Limpie la tapa del vial con la suspensión reconstituida con un hisopo estéril impregnado en alcohol.
- Coloque y sujete el vial con la suspensión reconstituida sobre una superficie dura. Encaje el adaptador del vial sujetando la parte externa del adaptador del vial y empujando la punta del adaptador del vial firmemente, a través del tapón de caucho, hasta que encaje en su lugar.
- Extraiga lentamente el volumen recomendado del vial con la jeringa luer-lock para poder inyectarlo. En el vial quedará una pequeña cantidad, un exceso de la suspensión.

Paso 4: Procedimiento de inyección
- Saque la jeringa luer-lock que contiene el volumen recomendado de la suspensión reconstituida de Abilify Maintena del vial.
- Seleccione una de las agujas hipodérmicas de seguridad descritas a continuación, en función del lugar de inyección y del peso del paciente, y una la aguja hipodérmica a la jeringa luer-lock que contiene la suspensión para inyectar. Asegúrese de que la aguja hipodérmica está firmemente unida al dispositivo protector de la aguja, presionando y girando en el sentido de las agujas del reloj, luego retire la cubierta de la aguja inmediatamente.
Tipo morfológico | Lugar de la inyección | Tamaño de la aguja |
No obesos | Deltoides | 25 mm calibre 23 |
Glúteo | 38 mm calibre 22 | |
Obesos | Deltoides | 38 mm calibre 22 |
Glúteo | 51 mm calibre 21 |
- Inyecte lentamente el volumen recomendado mediante una inyección intramuscular de una única dosis en el glúteo o el deltoides. No masajee el sitio de la inyección. Se debe tener cuidado y evitar inyectarla por error en un vaso sanguíneo. No inyecte en zonas con signos de inflamación, piel dañada, hinchazón y/o hematomas.
Sólo para inyección intramuscular profunda en el glúteo o el deltoides.
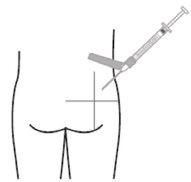
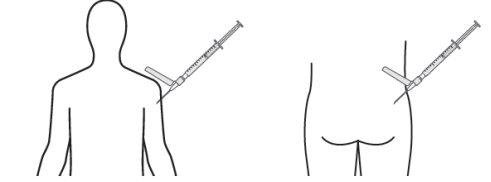
Deltoides Glúteo
Recuerde rotar los sitios de inyección en ambos glúteos y deltoides. Si se inicia el tratamiento con dos inyecciones, administre las inyecciones en dos sitios diferentes en dos músculos diferentes. NO inyecte ambas inyecciones de forma conjunta en el mismo deltoides o músculo glúteo.
En el caso de los metabolizadores lentos de CYP2D6 conocidos, administre en dos músculos deltoides separados o en un músculo deltoides y un músculo glúteo. NO inyecte en dos músculos glúteos.
Busque signos o síntomas de administración intravenosa involuntaria.
Paso 5: Procedimientos después de la inyección
Encaje el dispositivo de seguridad de la aguja como se describe en el Paso 2 e). Deseche los viales, adaptador, agujas y jeringa, de forma adecuada, después de la inyección. Los viales con polvo y disolvente son para un solo uso.

- País de registro
- Precio medio en farmacia315.49 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ABILIFY MAINTENA 400 MG POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADAForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 1 mg/mlPrincipio activo: AripiprazolFabricante: Kern Pharma S.L.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 10 mgPrincipio activo: AripiprazolFabricante: Kern Pharma S.L.Requiere recetaForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: AripiprazolFabricante: Kern Pharma S.L.Requiere receta
Médicos online para ABILIFY MAINTENA 400 MG POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ABILIFY MAINTENA 400 MG POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











