ZYCLARA 3.75% CREAM

How to use ZYCLARA 3.75% CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Zyclara 3.75% Cream
imiquimod
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Zyclara and what is it used for
- What you need to know before you use Zyclara
- How to use Zyclara
- Possible side effects
- Storage of Zyclara
- Contents of the pack and other information
1. What is Zyclara and what is it used for
Zyclara 3.75% cream contains the active substance imiquimod.
This medicine is prescribed for the treatment of actinic keratosis in adults, which is an Immune Response Modifier (to stimulate the human immune system).
This medicine stimulates your own immune system to produce natural substances that help fight actinic keratosis.
Actinic keratoses appear as rough areas of skin present in people who have been exposed to a large amount of sunlight over their lifetime. These areas can be the same color as your skin, or be greyish, pink, red, or brown. They can be flat and scaly, or raised, rough, hard, and wart-like.
This medicine should only be used for actinic keratosis of the face or balding scalp, if your doctor has decided that it is the most suitable treatment for you.
2. What you need to know before you use Zyclara
Do not use Zyclara
- if you are allergic to imiquimod or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Zyclara:
- if you have previously used this medicine or a similar preparation at a different concentration.
- if you have autoimmune disorders.
- if you have had an organ transplant.
- if you have abnormal blood counts.
General instructions during treatment
- If you have recently undergone surgical or pharmacological treatment, wait until the area to be treated has healed before using this medicine.
- Avoid contact with eyes, lips, and nostrils. In case of accidental contact, remove the cream by washing the area with water.
- Use the cream only externally (on the skin of the face or balding scalp).
- Do not use more cream than recommended by your doctor.
- Do not cover the treated area with bandages or other dressings after applying Zyclara.
- If you develop excessive discomfort in the treated area, remove the cream with a neutral soap and water. Once the discomfort has subsided, you can resume treatment as recommended. The cream should not be applied more than once a day.
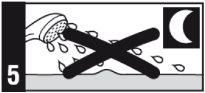
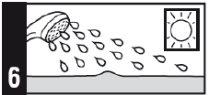
- Do not use sunlamps or tanning beds, and avoid sunlight as much as possible during treatment with this medicine. If you go outdoors during the day, use sunscreen and wear protective clothing, as well as a wide-brimmed hat.
Local skin reactions
While using Zyclara, you may experience local skin reactions due to the way the medicine works on your skin. These reactions may indicate that the medicine is working as expected.
During use of Zyclara and until healing, the treatment area may appear clearly different from normal skin. There is also a possibility that pre-existing inflammation may worsen temporarily.
This medicine may also cause flu-like symptoms (including fatigue, nausea, fever, muscle and joint pain, and chills) before or during the appearance of local skin reactions.
If you experience flu-like symptoms or a feeling of discomfort or intense local skin reactions, you may take a break of several days. You could resume treatment with imiquimod cream after the skin reaction has moderated. However, do not prolong the 2-week treatment cycle due to missed doses or rest periods.
The intensity of local skin reactions tends to be lower in the second cycle than in the first cycle of treatment with Zyclara.
The response to treatment cannot be adequately assessed until the resolution of local skin reactions. You should continue treatment as prescribed.
This medicine may reveal and treat actinic keratoses that have not been seen or perceived before, and which may subsequently disappear. Application should continue throughout the treatment cycle even if all actinic keratoses appear to have disappeared.
Children and adolescents
This medicine must not be used in children under 18 years, as the safety and efficacy in patients under this age have not been established. There are no data available on the use of imiquimod in children and adolescents.
Other medicines and Zyclara
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor before starting treatment if you are receiving immunosuppressive medicines that inhibit the immune system.
Avoid concomitant use of Zyclara and any other imiquimod cream on the same treatment area.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Your doctor will assess the risks and benefits of using Zyclara during pregnancy. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy.
It is unknown whether imiquimod passes into breast milk. You must not use Zyclara if you are breastfeeding or plan to breastfeed. Your doctor will decide whether you should stop breastfeeding or stop Zyclara treatment.
Driving and using machines
The influence of this medicine on the ability to drive and use machines is negligible.
Zyclara contains methylparaben, propylparaben, cetyl alcohol, stearyl alcohol, and benzyl alcohol.
It may cause allergic reactions (possibly delayed) because it contains methylparaben (E 218) and propylparaben (E 216).
This medicine may cause local skin reactions (such as contact dermatitis) because it contains cetyl alcohol and stearyl alcohol.
This medicine contains 5 mg of benzyl alcohol in each sachet. Benzyl alcohol may cause allergic reactions and moderate local irritation.
3. How to use Zyclara
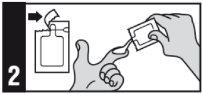
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again. Do not use this medicine until your doctor has shown you the correct way to do so.
This medicine should only be used for actinic keratosis of the face and balding scalp.
Dosage
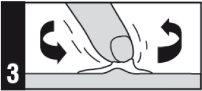
Apply this medicine to the affected area once a day, immediately before bedtime.
The maximum daily dose is 2 sachets (500 mg = 2 sachets of 250 mg each).
This medicine should not be applied to areas larger than the entire face or balding scalp.
Method of administration
|
|
|
|
|
|
|
|
|
|
|
|
Duration of treatment
Treatment starts with daily application for two weeks, followed by a two-week pause without application, and ends with daily application for two weeks again.
If you use more Zyclara than you should
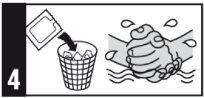
If you have applied too much cream, remove the excess by washing the area with water and a neutral soap.
Once any skin reaction has subsided, you can resume treatment according to the regular recommended schedule. The cream should not be applied more than once a day.
If you accidentally swallow this medicine, contact your doctor immediately.
If you forget to use Zyclara
If you miss a dose of Zyclara, wait until the next night to apply it and then continue with the usual routine. The cream should not be applied more than once a day. Each treatment cycle should not last more than two weeks, even if you have missed some doses.
If you stop using Zyclara
Consult your doctor before stopping treatment with Zyclara.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek medical attention immediately if you experience any of these serious side effects during use of this medicine:
Severe skin reactions (frequency not known), with skin lesions or spots on the skin that start as small red areas and evolve to resemble small targets, possibly with symptoms such as itching, fever, general malaise, joint pain, vision problems, burning sensation, pain or itching in the eyes, and mouth sores. If you experience these disorders, stop using this medicine and inform your doctor immediately.
In some people, a decrease in blood cell count (frequency not known) has been observed. This could increase your susceptibility to infections, develop bruising, or cause fatigue. If you notice any of these symptoms, inform your doctor.
Some patients with autoimmune disorders may experience worsening of their disease. Consult your doctor if you experience any changes during treatment with Zyclara.
If there is pus or other signs of infection on the skin (frequency not known), consult your doctor.
Many of the side effects of this medicine are due to its local action on the skin. Local skin reactions may indicate that the medicine is working as expected. If your skin reacts badly or you experience excessive discomfort during use of this medicine, stop the application of the cream and wash the area with water and a neutral soap. Then, contact your doctor or pharmacist, who may advise you to interrupt the application of this medicine for a few days (i.e., take a short break during treatment).
The following side effects have been reported with imiquimod:
Very common(may affect more than 1 in 10 people)
- Redness of the skin, crusts, skin scaling, suppuration, dryness of the skin, skin swelling, skin ulcers, and reduction of skin pigmentation at the application site.
Common(may affect up to 1 in 10 people)
- Other reactions at the application site, e.g., skin inflammation, itching, pain, burning sensation, irritation, and skin rash
- Swollen glands
- Headaches
- Dizziness
- Loss of appetite
- Nausea
- Diarrhea
- Vomiting
- Flu-like symptoms
- Fever
- Pain
- Muscle and joint pain
- Chest pain
- Insomnia
- Fatigue
- Viral infection (herpes simplex)
- Increased blood glucose
Uncommon(may affect up to 1 in 100 people)
- Changes in the application area, e.g., bleeding, small swollen areas on the skin, inflammation, tingling, increased sensitivity to touch, scarring, sensation of heat, skin lesions, blisters, or pustules
- Weakness
- Chills
- Lack of energy (lethargy)
- Discomfort
- Swelling of the face
- Back pain
- Limb pain
- Stuffy nose
- Sore throat
- Eye irritation
- Swelling of the eyelids
- Depression
- Irritability
- Dry mouth
- Abdominal pain
Rare(may affect up to 1 in 1,000 people)
- Flares of autoimmune diseases (an autoimmune disease is a disease caused by an abnormal immune response)
- Skin reactions distant from the application site
Frequency not known(cannot be estimated from the available data)
- Changes in skin color
Some patients have experienced changes in skin color in the area where Zyclara was applied. Although these changes tend to improve over time, in some patients they may be permanent
- Hair loss
A small number of patients have experienced hair loss in the treatment area or immediately adjacent to it
- Elevation of liver enzymes
There have been reports of an increase in liver enzymes.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
.
5. Storage of Zyclara
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the outer packaging after EXP.
The expiry date is the last day of the month stated.
Do not store above 25°C.
Once opened, the sachets should not be reused.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Zyclara Composition
- The active ingredient is imiquimod. Each sachet contains 9.375 mg of imiquimod in 250 mg of cream (100 mg of cream contain 3.75 mg of imiquimod).
- The other components are: isostearyl acid, benzyl alcohol, cetyl alcohol, stearic alcohol, light liquid paraffin, polysorbate 60, sorbitan stearate, glycerol, methylparaben (E 218), propylparaben (E 216), xanthan gum, purified water (see also section 2 "Zyclara contains methylparaben, propylparaben, cetyl alcohol, stearic alcohol and benzyl alcohol").
Appearance of Zyclara and Package Contents
- Each Zyclara 3.75% cream sachet contains 250 mg of a white to slightly yellowish cream, of uniform appearance.
- Each package contains 14, 28 or 56 single-use sachets of white low-density polyethylene/polyester/aluminum foil.
Only some package sizes may be marketed.
Marketing Authorization Holder
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart
Dublin 15
DUBLIN
Ireland
Manufacturer
Swiss Caps GmbH
Grassingerstraße 9
83043 Bad Aibling
Germany
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Mylan EPD bvba/sprl Terhulpsesteenweg, 6A B-1560 Hoeilaart Tel: +32 2 658 61 00 | Luxembourg Mylan EPD bvba/sprl Terhulpsesteenweg, 6A B-1560 Hoeilaart Tel: +32 2 658 61 00 |
Bulgaria Pharmaserve Ltd. Tel: +359 2 44 55 400 | Hungary Mylan EPD Kft. 1138 Budapest Váci út 150 Tel: +36 1 465 2100 |
Czech Republic Viatris CZ s.r.o. Tel: +420 222 004 400 | Malta V.J. Salomone Pharma Limited Upper Cross Road Marsa, MRS 1542 Tel: +356 21 22 01 74 |
Denmark Viatris ApS Borupvang 1 2750 Ballerup Tlf: +45 28 11 69 32 | Netherlands Mylan Healthcare B.V. Krijgsman 20 1186 DM Amstelveen Tel: +31 (0)20 426 3300 |
Germany Viatris Healthcare GmbH Lütticher Straße 5 53842 Troisdorf Tel: +49 800 0700 800 | Norway Viatris AS Hagaløkkveien 26 1383 Asker Tlf: +47 66 75 33 00 |
Estonia Meda Pharma SIA Liivalaia 13/15 11018 Tallinn Tel: +372 62 61 025 | Austria Mylan Österreich GmbH Guglgasse 15 1110 Wien Tel: +43 (0)1 86 390 0 |
Greece Viatris Hellas Ltd Tel: +30 210 010 0002 | Poland Mylan Healthcare Sp. z.o.o. ul. Postepu 21B 02-676 Warszawa Tel: +48 22 546 6400 |
Spain Viatris Pharmaceuticals, S.L.U. Tel: +34 900 102 712 | Portugal Viatris Healthcare, Lda. Av. D. João II, Edifício Atlantis, nº 44C – 7.3 e 7.4 1990-095 Lisboa Tel: +351 214 127 200 |
France Viatris Médical 1 bis place de la Défense – Tour Trinity 92400 Courbevoie Tél: +33 (0)1 40 80 15 55 | Romania BGP PRODUCTS SRL Tel.: +40 372 579 000 |
Croatia Viatris Hrvatska d.o.o. Koranska 2 10 000 Zagreb Tel: +385 1 2350 599 | Slovenia Viatris d.o.o. Tel: +386 1 23 63 180 |
Ireland Mylan Ireland Limited Tel: +353 1 8711600 | Slovakia Viatris Slovakia s.r.o. Tel: +421 2 32 199 100 |
Iceland Icepharma hf. Phone: +354 540 8000 | Finland Viatris Oy Vaisalantie 2-8/Vaisalavägen 2-8 02130 Espoo/Esbo Phone/Tel: +358 20 720 9555 |
Italy Mylan Italia Via Vittor Pisani, 20 20124 Milano Tel: +39 0261246921 | Sweden Viatris AB Box 23033 104 35 Stockholm +46 (0) 8 630 19 00 |
Cyprus Varnavas Hadjipanayis Ltd Giannou Kranidioti Avenue 226 TK 2234, Latsia, Nicosia Tel.: +357 22207700 | United Kingdom (Northern Ireland) Mylan IRE Healthcare Limited Tel: +353 18711600 |
Latvia Meda Pharma SIA 101 Mukusalas str. Riga LV-1004 Tel: +371 67616137 | |
Lithuania Meda Pharma SIA Žalgirio str. 90-100 Vilnius LT-09303 Tel: +370 52051288 |
Date of Last Revision of this Leaflet: (MM/YYYY)
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price66.11 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZYCLARA 3.75% CREAMDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: CREAM, 5 %Active substance: imiquimodManufacturer: Mibe Pharma Espana S.L.Prescription required
Online doctors for ZYCLARA 3.75% CREAM
Discuss questions about ZYCLARA 3.75% CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions