TAKHZYRO 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar TAKHZYRO 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
TAKHZYRO150mg solución inyectable en jeringa precargada
lanadelumab
Lea todo el prospecto detenidamente antes de administrar este medicamento a un niño/a, porque contiene información importante.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte al médico, farmacéutico o enfermero del niño/a.
- Este medicamento se le ha recetado solamente a su hijo/a o al niño/a que está a su cuidado, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que su hijo/a o el niño/a que está a su cuidado, ya que puede perjudicarles.
- Si el niño/a experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es TAKHZYRO y para qué se utiliza
- Qué necesita saber antes de administrar TAKHZYRO
- Cómo usar TAKHZYRO
- Posibles efectos adversos
- Conservación de TAKHZYRO
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es TAKHZYRO y para qué se utiliza
TAKHZYRO contiene el principio activo lanadelumab.
Para qué se utiliza TAKHZYRO
TAKHZYRO 150 mg es un medicamento que se utiliza en pacientes a partir de 2 años de edad con un peso corporal inferior a 40 kg con angioedema hereditario (AEH) para prevenir las crisis de angioedema.
Qué es el angioedema hereditario (AEH)
El AEH es una enfermedad hereditaria dentro de una misma familia. Cuando se tiene esta enfermedad, no hay en la sangre una cantidad suficiente de una proteína llamada «C1 inhibidor», o bien el C1 inhibidor no funciona correctamente. Esto lleva a un exceso de «calicreína plasmática», lo que a su vez produce niveles más altos de «bradicinina» en el torrente sanguíneo. Demasiada bradicinina provoca síntomas de AEH, como hinchazón y dolor en:
- las manos y los pies
- la cara, los párpados, los labios o la lengua
- las cuerdas vocales (laringe), que puede hacer que le cueste respirar
- los genitales
Cómo actúa TAKHZYRO
TAKHZYRO es un tipo de proteína que bloquea la actividad de la calicreína plasmática, lo que ayuda a reducir la cantidad de bradicinina presente en el torrente sanguíneo y previene los síntomas del AEH.
2. Qué necesita saber antes de empezar a usar TAKHZYRO
No use TAKHZYRO
Si su hijo/a o el niño/a que está a su cuidado es alérgico al lanadelumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
- Consulte al médico, farmacéutico o enfermero del niño/a antes de empezar a usar TAKHZYRO.
- Si tiene una reacción alérgica grave a TAKHZYRO con síntomas como erupción, opresión en el pecho, silbidos al respirar o latidos cardiacos rápidos, informe al médico, farmacéutico o enfermero del niño/a inmediatamente.
Llevar un registro
Se recomienda encarecidamente que, cada vez que su hijo/a o el niño/a que está a su cuidado reciban una dosis de TAKHZYRO, apunte el nombre y el número de lote del medicamento, para que tenga un registro de los lotes utilizados.
Pruebas analíticas
Informe al médico del niño/a de que está recibiendo TAKHZYRO antes de someter al niño/a a pruebas analíticas para evaluar su coagulación sanguínea, ya que la presencia de TAKHZYRO en la sangre puede interferir con ciertas pruebas analíticas y dar lugar a resultados inexactos.
Niños
No se recomienda el uso de TAKHZYRO en niños menores de 2 años, ya que no se ha estudiado en este grupo de edad.
Otros medicamentos y TAKHZYRO
Informe al médico o farmacéutico del niño/a si el niño/a está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No se sabe que TAKHZYRO afecte a otros medicamentos ni que se vea afectado por otros medicamentos.
Embarazo y lactancia
Pacientes que están embarazadas o en periodo de lactancia o creen que podrían estar embarazadas o tienen intención de quedarse embarazadas debe consultar a su médico o farmacéutico antes de utilizar este medicamento. La información sobre la seguridad del uso de TAKHZYRO durante el embarazo y la lactancia es limitada. Como medida de precaución, es preferible evitar el uso de lanadelumab durante el embarazo y la lactancia. El médico analizará con usted los riesgos y beneficios de recibir este medicamento.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
TAKHZYRO contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por jeringa precargada; esto es, esencialmente “exento de sodio”.
3. Cómo usar TAKHZYRO
TAKHZYRO se presenta en jeringas precargadas de un solo uso como solución lista para usar. Un médico con experiencia en la atención de pacientes con AEH iniciará y supervisará el tratamiento de su hijo/a o del niño/a que está a su cuidado.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por el médico, farmacéutico o enfermero del niño/a. En caso de duda u otras preguntas sobre el uso de este medicamento, pregunte al médico, farmacéutico o enfermero del niño/a.
Cuánto TAKHZYRO utilizar
Para niños de 2 años a menores de 12 años de edad, la dosis de inicio recomendada se basa en el peso corporal:
Peso corporal (kg) | Dosis de inicio recomendada | Ajuste de dosis |
10 a menos de 20 kg | 150 mg de lanadelumab cada 4 semanas | Se puede considerar un aumento de la dosis a 150 mg de lanadelumab cada 3 semanas en pacientes con control insuficiente de los ataques |
20 a menos de 40 kg | 150 mg de lanadelumab cada 2 semanas | Se puede considerar reducir la dosis a 150 mg de lanadelumab cada 4 semanas en pacientes estables y sin crisis bajo tratamiento |
40 kg o más | 300 mg de lanadelumab cada 2 semanas | Se puede considerar reducir la dosis a 300 mg de lanadelumab cada 4 semanas en pacientes estables y sin crisis bajo tratamiento |
- En pacientes con un peso corporal de 20 a menos de 40 kg que no hayan tenido ninguna crisis durante un periodo de tiempo prolongado, el médico puede permitir que su hijo/a o el niño/a que está a su cuidado continúe recibiendo la misma dosis al llegar a los 12 años de edad.
Para adultos y adolescentes de 12 años a menores de 18 años de edad con un peso corporal inferior a 40 kg:
- La dosis de inicio recomendada es de 300 mg de lanadelumab cada 2 semanas. Si no ha tenido ninguna crisis durante un periodo de tiempo prolongado, el médico puede modificarle la dosis a 300 mg de lanadelumab cada 4 semanas, en especial si su peso corporal es bajo.
- También puede considerarse una dosis de inicio de 150 mg de lanadelumab cada 2 semanas. Si no ha tenido crisis durante un periodo de tiempo prolongado, el médico puede cambiar la dosis a 150 mg de lanadelumab cada 4 semanas.
Cómo inyectar TAKHZYRO
TAKHZYRO debe ser inyectado por un profesional sanitario o un cuidador. El cuidador debe leer y seguir atentamente las instrucciones que aparecen en la sección 7, «Instrucciones de uso».
- TAKHZYRO se inyecta bajo la piel («inyección subcutánea»).
- La inyección puede ser administrada por un profesional sanitario o un cuidador.
- Un médico, farmacéutico o enfermero deberá enseñarle a preparar e inyectar TAKHZYRO correctamente antes de que lo administre por primera vez. No lleve a cabo la inyección hasta que le hayan enseñado a inyectar el medicamento.
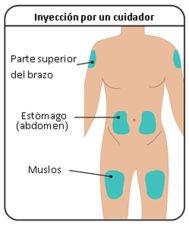
- Inserte la aguja en el tejido adiposo del estómago (abdomen), muslo o parte superior del brazo.
- Inyecte el medicamento en un lugar distinto cada vez.
- Use cada jeringa precargada de TAKHZYRO solo una vez.
Si usa más TAKHZYRO del que debe
Informe al médico, farmacéutico o enfermero del niño/a si el niño/a ha recibido una dosis de TAKHZYRO mayor a la recomendada o antes de lo prescrito por el médico.
Si olvidó usar TAKHZYRO
Si se salta una dosis de TAKHZYRO, inyecte la dosis lo antes posible. La administración de las siguientes dosis puede requerir un ajuste según la frecuencia de dosificación deseada, asegurándose de:
- dejar pasar al menos 10 días entre una dosis y otra en pacientes con un régimen de dosificación cada 2 semanas,
- dejar pasar al menos 17 días entre una dosis y otra en pacientes con un régimen de dosificación cada 3 semanas,
- dejar pasar al menos 24 días entre una dosis y otra en pacientes con un régimen de dosificación cada 4 semanas.
Si no está seguro de cuándo inyectar TAKHZYRO después de haberse saltado una dosis, pregunte al médico, farmacéutico o enfermero del niño/a.
Si interrumpe el tratamiento con TAKHZYRO
La decisión de interrumpir la administración de TAKHZYRO debe consultarse con el médico del niño/a, ya que los síntomas podrían regresar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte al médico, farmacéutico o enfermero del niño/a.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si el niño/a tiene una reacción alérgica grave a TAKHZYRO con síntomas como erupción, opresión en el pecho, silbidos al respirar o latidos cardiacos rápidos, informe al médico, farmacéutico o enfermero del niño/a inmediatamente.
Informe al médico, farmacéutico o enfermero del niño/a si usted o el niño/a notan alguno de los siguientes efectos adversos.
Muy frecuentes (pueden afectar a más de 1de cada 10personas):
- Reacciones en el lugar de administración de la inyección: los síntomas son dolor, enrojecimiento de la piel, cardenales, molestias, hinchazón, sangrado, picor, endurecimiento de la piel, hormigueo, calor y erupción.
Frecuentes (pueden afectar hasta 1de cada 10personas):
- Reacciones alérgicas, como picor, molestias y hormigueo en la lengua
- Mareos, sensación de desmayo
- Erupción cutánea abultada
- Dolor muscular
- Resultados de pruebas analíticas que muestran cambios en el hígado
Comunicación de efectos adversos
Si su hijo/a o el niño/a que está a su cuidado experimenta cualquier tipo de efecto adverso, consulte al médico, farmacéutico o enfermero del niño/a, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de TAKHZYRO
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
TAKHZYRO 150 mg solución inyectable en jeringa precargada.
Conservar en nevera (entre 2 ?C y 8 ?C). No congelar. Conservar la jeringa precargada en el embalaje exterior para protegerla de la luz.
Las jeringas precargadas pueden conservarse por debajo de los 25 °C por un solo periodo de 14 días, pero sin sobrepasar la fecha de caducidad.
Después de su conservación a temperatura ambiente, no volver a refrigerar TAKHZYRO para su conservación.
Cuando se retira de la refrigeración una jeringa precargada de un envase múltiple, se deberán volver a colocar las jeringas precargadas restantes en la nevera para cuando haya que usarlas en un futuro.
No utilice este medicamento si observa signos de deterioro, como partículas en la jeringa precargada o un cambio de color en la solución para inyección.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de TAKHZYRO
- El principio activo es lanadelumab. Cada jeringa precargada contiene 150 mg de lanadelumab en solución de 1 ml.
- Los demás componentes son fosfato disódico dihidratado, ácido cítrico monohidratado, histidina, cloruro de sodio, polisorbato 80 y agua para preparaciones inyectables; ver sección 2 «TAKHZYRO contiene sodio»
Aspecto del producto y contenido del envase
TAKHZYRO se presenta en forma de solución inyectable transparente de incolora a amarillo pálido en una jeringa precargada.
TAKHZYRO está disponible como:
- envase unitario que contiene una jeringa precargada de 1 ml en una caja
- envase unitario que contiene dos jeringas precargadas de 1 ml en una caja
- envases múltiples que contienen 3 cajas intermedias, cada una de ellas con dos jeringas precargadas de 1 ml.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Takeda Pharmaceuticals International AG Ireland Branch
Block 2 Miesian Plaza
50-58 Baggot Street Lower
Dublín 2
D02 HW68
Irlanda
Responsable de la fabricación
Takeda Pharmaceuticals International AG Ireland Branch
Block 2 Miesian Plaza
50-58 Baggot Street Lower
Dublin 2
D02 HW68
Irlanda
Shire Pharmaceuticals Ireland Limited
Blocks 2 & 3 Miesian Plaza
50-58 Baggot Street Lower
Dublín 2
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Takeda Belgium NV Tél/Tel: +32 2 464 06 11 | Lietuva Takeda, UAB Tel: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tél/Tel: +32 2 464 06 11 |
Ceská republika Takeda Pharmaceuticals Czech Republic s.r.o. Tel: + 420 234 722 722 | Magyarország Takeda Pharma Kft. Tel.: +36 1 270 7030 |
Danmark Takeda Pharma A/S Tlf.: +45 46 77 10 10 | Malta Drugsales Ltd Tel: +356 21419070 |
Deutschland Takeda GmbH Tel: +49 (0)800 825 3325 | Nederland Takeda Nederland B.V. Tel: +31 20 203 5492 |
Eesti Takeda Pharma OÜ Tel: +372 6177 669 | Norge Takeda AS Tlf.: +47 800 800 30 |
Ελλ?δα Τakeda ΕΛΛΑΣ Α.Ε. Tηλ: +30 210 6387800 | Österreich Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 |
España Takeda Farmacéutica España, S.A. Tel: +34 917 90 42 22 | Polska Takeda Pharma Sp. z o.o. Tel.: +48223062447 |
France Takeda France SAS Tél : + 33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: + 351 21 120 1457 |
Hrvatska Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | România Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd Tel: 1800 937 970 | Slovenija Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel: + 386 (0) 59 082 480 |
Ísland Vistor ehf. Sími: +354 535 7000 | Slovenská republika Takeda Pharmaceuticals Slovakia s.r.o. Tel: +421 (2) 20 602 600 |
Italia Takeda Italia S.p.A. Tel: +39 06 502601 | Suomi/Finland Takeda Oy Puh/Tel: 0800 774 051 |
Κ?προς Proton Medical (Cyprus) Ltd Τηλ: +357 22866000 | Sverige Takeda Pharma AB Tel: 020 795 079 |
Latvija Takeda Latvia SIA Tel: +371 67840082 | United Kingdom (Northern Ireland) Takeda UK Ltd Tel: +44 (0) 3333 000 181 |
Fecha de la última revisión de este prospecto
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- Instrucciones de uso
Asegúrese de leer, comprender y seguir las instrucciones de uso antes de inyectar TAKHZYRO. Contacte con un profesional sanitario si tiene preguntas.
Uso previsto
La jeringa precargada de TAKHZYRO es un dispositivo de inyección con aguja, desechable, listo para su uso con una dosis fija (150 mg/1 ml) destinado a la administración subcutánea del medicamento por profesionales sanitarios y cuidadores. La autoadministración no está recomendada en pacientes pediátricos (2años a menores de 12años).
Conservación de TAKHZYRO
- Guarde la jeringa precargada de TAKHZYRO en la nevera a 2 °C a 8 °C. Nocongelar.
- Una jeringa precargada que se retire de la refrigeración debe conservarse a menos de 25 °C y utilizarse en un plazo de 14 días. Después de su conservación a temperatura ambiente, no volver a refrigerar TAKHZYRO para su conservación.
- Cuando se retira de la refrigeración una jeringa precargada de un envase múltiple, se deberán volver a colocar las jeringas precargadas restantes en la nevera para cuando haya que usarlas en un futuro.
- Guarde TAKHZYRO en la caja original para proteger la jeringa precargada de la luz.
- Deseche la jeringa precargada de TAKHZYRO si no se ha refrigerado, se ha congelado o no se ha guardado en su envase original protegida de la luz.
- No agiteTAKHZYRO.
Mantenga TAKHZYRO y todos los medicamentos fuera del alcance de los niños.
Partes de su jeringa precargada de TAKHZYRO antes de su uso (FiguraA).
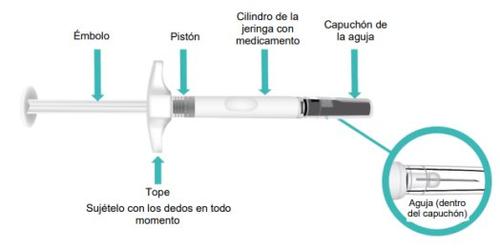
Figura A: Jeringa precargada de TAKHZYRO
Paso1: Preparación de la inyección
- Tome una toallita con alcohol, una almohadilla de gasa o torunda de algodón, una venda adhesiva y un contenedor para desechar objetos punzocortantes (FiguraB) y colóquelo todo en una superficie plana y limpia en una zona bien iluminada. Estos productos no se incluyen en el envase de TAKHZYRO.

Figura B: Suministros
15 minutos antes de inyectar.
|
|
extraiga la jeringa precargada de TAKHZYRO de la bandeja (Figura C).
esté listo para comenzar la inyección.
esté listo para comenzar la inyección. |
|
Figura C: Extraer la jeringa precargada
Séquese completamente las manos.
cuerpo después de lavarse las manos antes de comenzar la inyección. |
|
Figura D: Lavado de manos
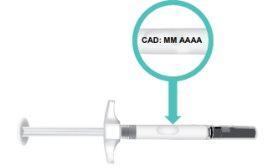
en el cilindro de la jeringa (Figura E).
TAKHZYRO si la fecha de caducidad ha pasado. Si la jeringa precargada está caducada, deséchela en un contenedor para objetos punzocortantes y contacte con su profesional sanitario. |
|
Figura E: Localización de la fecha de
caducidad
TAKHZYRO para comprobar que no esté dañada. El medicamento del interior del cilindro de la jeringa debe ser incoloro o amarillo pálido. (Figura F).
TAKHZYRO si la jeringa está dañada o tiene grietas.
TAKHZYRO si el medicamento ha perdido el color, está turbio o presenta partículas o residuos.
aire en su jeringa precargada de TAKHZYRO. Es algo normal y no afectará a la dosis. Si no puede utilizar la jeringa precargada, contacte con su profesional sanitario. |
Figura F: Examinar la jeringa precargada |
Paso2: Selección y preparación del lugar de inyección
del cuerpo del niño/a donde la piel esté irritada, enrojecida, con hematomas o infectada.
debe estar como mínimo a una distancia de 5 cm de cualquier cicatriz o del ombligo. Importante: Rotar los lugares de inyecciónpara mantener la piel sana. Cada nueva inyección debe administrarse a una distancia como mínimo de 3 cm del último lugar que utilizó. |
Figura G: Lugares de inyección |
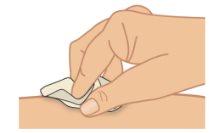
toallita con alcohol y espere a que se seque (Figura H).
ha limpiado.
administrar la inyección. |
Figura H: Limpiar el lugar de inyección |
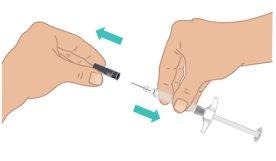
aguja con una mano mientras sujeta firmemente la parte central de la jeringa precargada de TAKHZYRO con la otra. Deseche el capuchón de la aguja (Figura I).
hasta que esté listo para comenzar la inyección.
TAKHZYRO si se ha dejado caer sin el capuchón de la aguja.
TAKHZYRO si la aguja parece dañada o torcida.
aguja entre en contacto con nada. Es posible que vea burbujas de aire, es normal. Nointente quitar las burbujas. |
Figura I: Quitar el capuchón de la aguja |
- Deseche el capuchón de la aguja en la papelera o en el contenedor para objetos punzocortantes.
- Para evitar herirse con la aguja, novuelva a tapar la aguja.
Paso3: Inyección de TAKHZYRO
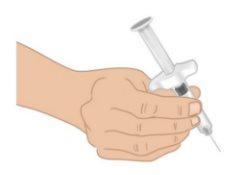
TAKHZYRO con una mano como si fuera un lápiz (FiguraJ). Evite tocar la aguja o empujar el émbolo. |
Figura J: Sujetar la aguja precargada |
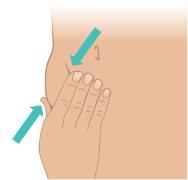
cuidado la piel, formando un pliegue de unos 3 cm en el lugar de la inyección que ha limpiado. Mantenga el pliegue hasta que la inyección haya terminado y se haya quitado la aguja (Figura K). |
Figura K: Pellizcar un pliegue en la piel de 3 cm |
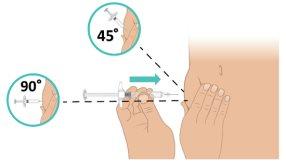
rápido, inserte la aguja en la piel en un ángulo de 45 a 90 grados. Asegúrese de que mantiene la aguja en su lugar (Figura L). Importante:Inyectar directamente en el tejido adiposo bajo la piel (inyección subcutánea). |
Figura L: Insertar la aguja |
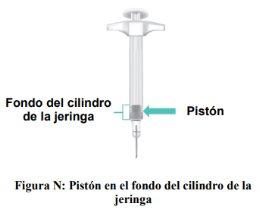
Importante: Noretire la aguja hasta que se haya inyectado todo el líquido y la jeringa se quede vacía. Cuando la inyección se complete, el pistón estará en el fondo del cilindro de la jeringa (Figura N).
|
Figura M: Empujar el émbolo hasta que se detenga |
| |
|
Paso 4: Desechar la jeringa precargada de
TAKHZYRO
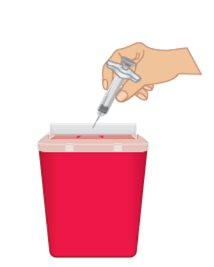
un contenedor para desechar objetos punzocortantes inmediatamente después de utilizarla (Figura O).
tapar la aguja.
TAKHZYRO ni ningún otro producto empleado para la inyección.
TAKHZYRO en la basura.
para desechar objetos punzocortantes fuera del alcance de los niños. |
Figura O: Desechar en un contenedor para objetos punzocortantes |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TAKHZYRO 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 300 mgPrincipio activo: LanadelumabRequiere recetaForma farmacéutica: INYECTABLE, 300 mgPrincipio activo: LanadelumabRequiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: Drugs used in hereditary angioedemaFabricante: Csl Behring GmbhRequiere receta
Médicos online para TAKHZYRO 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TAKHZYRO 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes