ANDEMBRY 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA


Cómo usar ANDEMBRY 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ANDEMBRY 200 mg solución inyectable en pluma precargada
garadacimab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta cualquier tipo de efecto secundario, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es ANDEMBRY y para qué se utiliza
- Qué necesita saber antes de empezar a usar ANDEMBRY
- Cómo usar ANDEMBRY
- Posibles efectos adversos
- Conservación de ANDEMBRY
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es ANDEMBRY y para qué se utiliza
ANDEMBRY contiene el principio activo garadacimab.
ANDEMBRY es un medicamento utilizado en pacientes a partir de 12 años de edad con angioedema hereditario (AEH) para prevenir las crisis de angioedema.
El AEH es una enfermedad que provoca episodios recurrentes de hinchazón de rápida aparición, conocidos como crisis de AEH, en distintas partes del cuerpo, incluidos:
- manos y pies;
- cara, párpados, labios o lengua;
- laringe y garganta, lo que puede dificultar la respiración;
- genitales;
- estómago e intestino.
Las crisis de AEH pueden ser dolorosas e incapacitantes. Las crisis que afectan a la garganta o la laringe pueden ser peligrosas o incluso poner en peligro la vida.
Aunque el AEH a menudo se presenta en familias, algunas personas pueden no tener antecedentes familiares. Se conocen tres tipos de AEH, según el tipo de defecto genético y su efecto sobre una proteína que circula por la sangre, denominada inhibidor de la C1 esterasa (C1-INH). Una persona puede tener niveles bajos de C1-INH en el organismo (AEH tipo I), C1-INH de funcionamiento deficiente (AEH tipo II) o AEH con C1-INH de funcionamiento normal (AEH tipo III). El último tipo es extremadamente raro. Los tres tipos producen los mismos síntomas clínicos de hinchazón localizada.
El C1-INH regula un proceso en el organismo que controla la producción de una sustancia inflamatoria denominada bradicinina. La sobreproducción de bradicinina provoca hinchazón e inflamación en personas con AEH.
El principio activo de ANDEMBRY, garadacimab, bloquea la activación de una proteína conocida como factor XIIa (FXIIa), que participa en la estimulación de la producción de bradicinina. Al bloquear la actividad del FXIIa, garadacimab reduce el nivel de bradicinina, para prevenir así los ataques de AEH. Algunas subcategorías de AEH con C1-INH normal pueden no responder al tratamiento con garadacimab. Hable con su médico si tiene alguna preocupación acerca de su medicamento.
2. Qué necesita saber antes de empezar a usar ANDEMBRY
No use ANDEMBRY
Si es alérgico a garadacimab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
- Consulte a su médico, farmacéutico o enfermero antes de empezar a usar ANDEMBRY.
- Si tiene una reacción alérgica grave a ANDEMBRY con síntomas como urticaria, opresión en el pecho, dificultad para respirar, sibilancias, hipotensión o anafilaxia, informe a su médico, farmacéutico o enfermero inmediatamente.
- Trate una crisis de angioedema hereditario con su medicamento de rescate habitual sin tomar dosis adicionales de ANDEMBRY.
Mantenga un registro
Se recomienda encarecidamente que, cada vez que se administre ANDEMBRY, anote el nombre y el número de lote del medicamento. De este modo mantendrá un registro de los lotes utilizados.
Pruebas analíticas
Informe a su médico si está usando ANDEMBRY antes de realizarse pruebas analíticas para medir la coagulación de su sangre. Esto se debe a que ANDEMBRY puede interferir con algunas pruebas analíticas y dar lugar a resultados inexactos.
Niños y adolescentes
No se recomienda el uso de ANDEMBRY en niños menores de 12 años de edad. Esto se debe a que no se ha estudiado en este grupo de edad.
Otros medicamentos y ANDEMBRY
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No se conoce que ANDEMBRY afecte a otros medicamentos ni que se vea afectado por otros medicamentos.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar ANDEMBRY. Existe información limitada sobre la seguridad del uso de ANDEMBRY durante el embarazo y la lactancia. Como medida de precaución, es preferible evitar el uso de ANDEMBRY durante el embarazo. Su médico discutirá con usted los riesgos y beneficios de tomar este medicamento.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
ANDEMBRY contiene prolina.
Este medicamento contiene 19,3 mg de prolina en cada pluma precargada, equivalente a 16,1 mg/ml. La prolina puede ser perjudicial para los pacientes con hiperprolinemia, una enfermedad genética rara en la que la prolina se acumula en el organismo. Si usted (o su hijo) padece hiperprolinemia, no utilice este medicamento a menos que su médico se lo recomiende.
ANDEMBRY contiene polisorbato 80.
Este medicamento contiene 0,24 mg de polisorbato 80 en cada pluma precargada, lo que equivale a 0,2 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene alguna alergia conocida.
3. Cómo usar ANDEMBRY
ANDEMBRY se presenta en una pluma precargada de un solo uso. Su tratamiento comenzará bajo la supervisión y gestión de un profesional sanitario.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico, farmacéutico o enfermero. En caso de duda o de cualquier otra pregunta sobre el uso de este medicamento, consulte de nuevo a su médico, farmacéutico o enfermero.
Cuánto ANDEMBRY usar
La dosis recomendada de ANDEMBRY es una dosis de carga inicial de 400 mg administrada en dos inyecciones de 200 mg el primer día de tratamiento, seguida de una dosis mensual de 200 mg.
Cómo inyectar ANDEMBRY
Puede autoadministrarse ANDEMBRY o un cuidador puede administrárselo. En ambos casos, usted o su cuidador deben leer y seguir atentamente las instrucciones de la sección 7, “Instrucciones de uso”.
- ANDEMBRY se administra de forma SC bajo la piel (“inyección subcutánea”) en la barriga (abdomen), el muslo o la parte superior del brazo.
- Un médico, farmacéutico o enfermero debe mostrarle cómo administrar ANDEMBRY correctamente antes de usarlo por primera vez. No se autoadministre ni permita que un cuidador le administre hasta no haber recibido formación sobre cómo inyectar el medicamento.
- Use cada pluma precargada solo una vez.
- Si la pluma no funciona como está previsto, informe a su médico, farmacéutico o enfermero lo antes posible.
- Se recomienda rotar entre lugares de inyección.
Si usa más ANDEMBRY del que debe
Informe a su médico, farmacéutico o enfermero si usa demasiado ANDEMBRY.
Si olvidó usar ANDEMBRY
Si olvida una dosis de ANDEMBRY, adminístrese su dosis lo antes posible. Si no está seguro de cuándo inyectar ANDEMBRY después de una dosis olvidada, consulte a su médico, farmacéutico o enfermero.
Si interrumpe el tratamiento con ANDEMBRY
Es importante que siga administrándose ANDEMBRY según las indicaciones de su médico, incluso si se siente mejor.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico, farmacéutico o enfermero si nota alguno de los siguientes efectos secundarios.
Frecuentes(pueden afectar hasta a 1 de cada 10 personas):
- Reacciones en el lugar de administración que incluyen enrojecimiento, hematomas, picor y urticaria
- Cefalea
- Dolor abdominal
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto secundario, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional incluido en el Apéndice V.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ANDEMBRY
Mantener este medicamento fuera de la vista y del alcance de los niños.
No use este medicamento después de la fecha de caducidad que aparece en la caja externa y en la etiqueta después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en el frigorífico (entre 2 °C - 8 °C). No congelar. Conservar la pluma precargada en el embalaje exterior para protegerla de la luz.
La pluma precargada podrá conservarse a temperatura ambiente (hasta 25 °C) durante un único período de hasta 2 meses, pero no más allá de la fecha de caducidad.
No vuelva a almacenar ANDEMBRY en el frigorífico después de haberlo conservado a temperatura ambiente.
No use este medicamento si observa signos de deterioro como partículas o cambio de color de la solución.
Los medicamentos no se deben desechar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ANDEMBRY
- El principio activo es garadacimab. Cada pluma precargada contiene 200 mg de garadacimab en 1,2 ml de solución.
- Los demás componentes son histidina, monohidrocloruro de arginina, prolina, polisorbato 80 y agua para preparaciones inyectables; ver sección 2 “ANDEMBRY contiene prolina y polisorbato 80”.
Aspecto de ANDEMBRY y contenido del envase
ANDEMBRY se presenta como una solución inyectable de color amarillo parduzco a amarillo, ligeramente opalescente a transparente, en una pluma precargada.
ANDEMBRY se presenta en envases individuales que contienen una pluma precargada de 1,2 ml y en envases múltiples de 3 estuches, cada uno de los cuales contiene 1 pluma precargada.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización y responsable de la fabricación
CSL Behring GmbH
Emil-von-Behring-Strasse 76
D-35041 Marburgo
Alemania
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien CSL Behring NV Tél/Tel: +32 15 28 89 20 | Lietuva CentralPharma Communications UAB Tel: +370 5 243 0444 |
| Luxembourg/Luxemburg CSL Behring NV Tél/Tel: +32 15 28 89 20 |
Ceská republika CSL Behring s.r.o. Tel: +420 702 137 233 | Magyarország CSL Behring Kft. Tel: +36 1 213 4290 |
Danmark CSL Behring AB Tlf: +46 8 544 966 70 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Deutschland CSL Behring GmbH Tel: +49 6190 75 84810 | Nederland CSL Behring BV Tel: +31 85 111 96 00 |
Eesti CentralPharma Communications OÜ Tel: +3726015540 | Norge CSL Behring AB Tlf: +46 8 544 966 70 |
Ελλáδα CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Österreich CSL Behring GmbH Tel: +43 1 80101 1040 |
España CSL Behring S. A. Tel: +34 933 67 1870 | Polska CSL Behring Sp. z o.o. Tel.: +48 22 213 22 65 |
Francia CSL Behring SA Tél: +33 1 53 58 54 00 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
Hrvatska Marti Farm d.o.o. Tel: +385 1 5588297 | România Prisum Healthcare S.R.L. Tel: +40 21 322 01 71 |
Ireland CSL Behring GmbH Tel: +49 69 305 17254 | Slovenija EMMES BIOPHARMA GLOBAL s.r.o. - podružnica v Sloveniji Tel: +386 41 42 0002 |
Ísland CSL Behring AB Sími: +46 8 544 966 70 | Slovenská republika CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Italia CSL Behring S.p.A. Tel: +39 02 34964 200 | Suomi/Finland CSL Behring AB Puh/Tel: +46 8 544 966 70 |
Κúπρος CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Sverige CSL Behring AB Tel: +46 8 544 966 70 |
Latvija CentralPharma Communications SIA Tel: +371 6 7450497 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- Instrucciones de uso
ANDEMBRY solución inyectable en
pluma precargada
uso subcutáneo
Importante:
Esta pluma precargada funciona de manera diferente a otros dispositivos de administración subcutáneo. Lea detenidamente las instrucciones de uso antes de usarlo y cada vez que reciba una nueva pluma precargada, ya que puede que haber información actualizada. Esta información no reemplaza la consulta con su profesional sanitario sobre su enfermedad o tratamiento. En pacientes adolescentes, ANDEMBRY debe administrarse bajo la supervisión de un adulto. Asegúrese de haber recibido formación por parte de su profesional sanitario antes de usar esta pluma precargada por primera vez. |
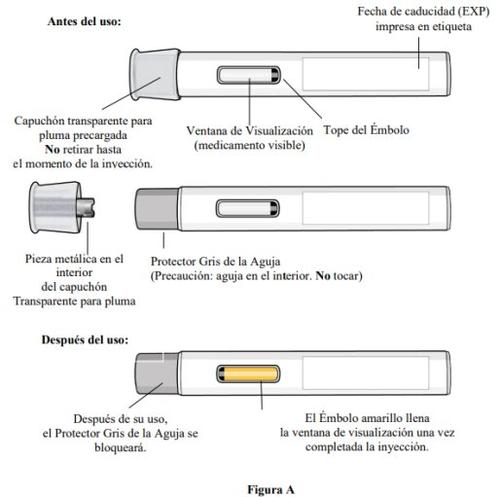
Partes de la pluma (ver figura A):
Continúe con las siguientes secciones para preparar y realizar la inyección.
Lea la siguiente información de seguridad:
- Mantenga la pluma precargada en su caja de cartón original hasta su uso, para protegerla de la luz.
- Noretire el capuchón transparente para pluma recargada hasta el momento de la inyección.
- Novuelva a colocar el capuchón transparente de la pluma precargada después de retirarlo, ya que podría iniciar la inyección y provocar lesiones.
- La pluma precargada contiene 1 dosis y es para un solo uso. Noreutilice la misma jeringa precargada.
- Nouse la pluma precargada si se ha superado la fecha de caducidad.
- La pluma precargada es solo para inyección subcutánea (debajo de la piel).
- Nouse la pluma precargada si parece dañada, tiene grietas, pierde medicamento o se ha caído. En estos casos deseche la pluma precargada tal como se describe en el paso 11y use una nueva.
- Noadministre el medicamento a través de la ropa.
- Notoque ni intente quitar el protector gris de la aguja en ningún momento.
- Mantener ANDEMBRY fuera de la vista y del alcance de los niños.
Póngase en contacto con el profesional sanitario que lo atiende si le surge alguna pregunta.
¿Cómo debo conservar ANDEMBRY?
- Conserve ANDEMBRY pluma precargada en frigorífico, entre 2 °C y 8 °C, en su caja original hasta su uso, para protegerlo de la luz.
- Nocongelar. Si la pluma precargada se ha congelado, no la useincluso si está descongelada.
- Saque la pluma precargada del frigorífico 30 minutos antes de su uso, para dejar así que alcance la temperatura ambiente.
Conservación alternativa (temperatura ambiente):
- En caso necesario, por ejemplo, cuando viaje, la pluma precargada podrá conservarse a temperatura ambiente (hasta 25 °C) durante un único período de hasta 2 meses, pero no más allá de la fecha de caducidad.
- Si decide conservar la pluma precargada a temperatura ambiente:
- En el espacio provisto en la caja de cartón, escriba la fecha en la que sacó por primera vez la pluma precargada del frigorífico para ayudarle a llevar un registro de cuánto tiempo ha estado conservada a temperatura ambiente.
- Novuelva a refrigerar la pluma precargada después de que haya alcanzado la temperatura ambiente.
- Deseche la pluma precargada si se ha conservado a temperatura ambiente durante más de 2 meses (ver paso 11. Eliminación de la pluma precargada).
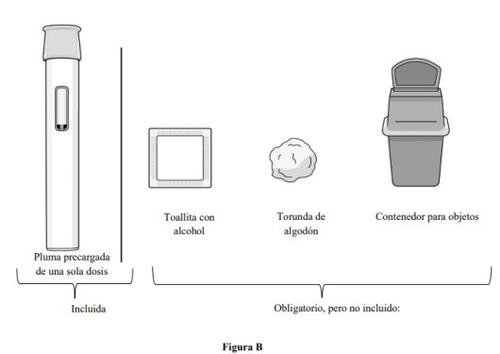
Suministros necesarios para la inyección con pluma precargada (ver figura B):
Incluida en la caja de cartón:
- 1 pluma precargada de una sola dosis
Suministros necesarios, pero no incluidos en la caja de cartón:
- 1 toallita con alcohol
- 1 torunda de algodón o una gasa
- 1 contenedor de objetos punzantes o resistente a la punción para su eliminación (ver el paso 11. Eliminación de la pluma precargada).
Preparación para la administración
No retire el capuchón transparente para pluma recargada hasta justo antes de la administración.
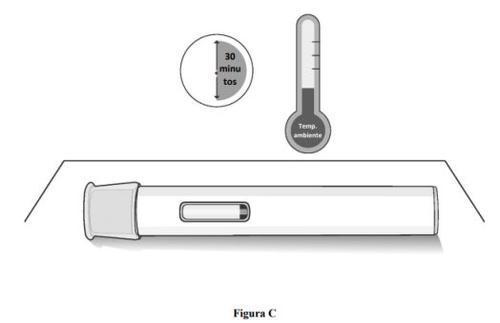
Paso 1. Deje que la pluma precargada alcance la temperatura ambiente.
- Retire la pluma precargada de la caja de cartón y colóquela sobre una superficie plana y limpia.
- Espere 30 minutosa que el medicamento alcance la temperatura ambiente si se ha conservado en el frigorífico (ver figura C).
- Inyectarse el medicamento frío podría provocarle molestias.
- Nointente acelerar el proceso de calentamiento de ninguna manera. Por ejemplo, nolo caliente en el microondas, en agua caliente ni lo deje expuesto a la luz solar directa.
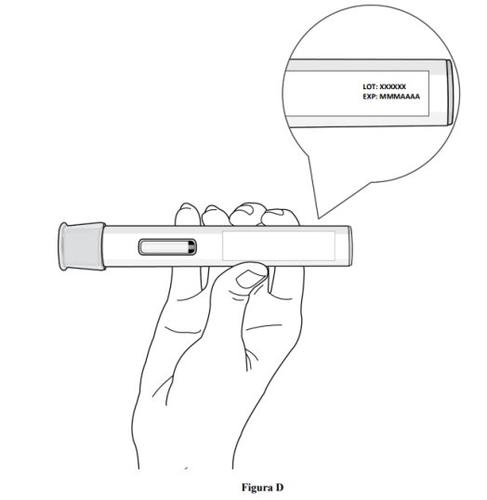
Paso 2. Compruebe la fecha de caducidad
- Compruebe la fecha de caducidad que figura en la etiqueta de la pluma precargada (ver figura D).
- No usela pluma precargada si se ha superado la fecha de caducidad.
- No usela pluma precargada si se ha conservado a temperatura ambiente durante más de 2 meses.
- Si se ha superado la fecha de caducidad o si se ha conservado a temperatura ambiente durante más de 2 meses, deseche la pluma precargada de forma segura y coja una nueva (ver paso 11. Eliminación de la pluma precargada).
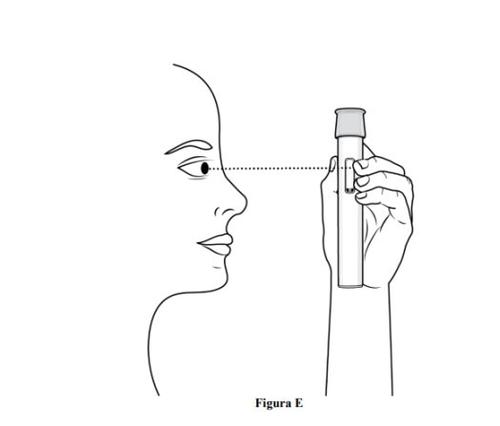
Paso 3. Inspeccione la pluma precargada y el medicamento
- Compruebeque la pluma precargada no esté dañada.
- Inspeccioneel medicamentomediante la ventana de visualización de la pluma precargada (ver figura E).
- Es normal ver burbujas de aire, nointente eliminarlas.
- El medicamento debe ser de color amarillo parduzco a amarillo y puede parecer ligeramente opalescente a transparente.
- No usela pluma precargada, deséchela de forma segura y coja una nueva (ver paso 11. Eliminación de la pluma precargada) en caso de que:
- El medicamento está descolorido o contenga partículas.
- La pluma precargada parezca dañada o presente grietas.
- La pluma precargada presente fugas.
- La pluma precargada se haya caído sobre una superficie dura, incluso si no parece dañada.
Elija y prepare un lugar de inyección
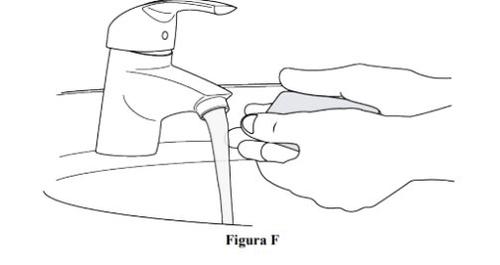
Paso 4. Lávese las manos.
- Lávese las manos con agua y jabón o use una solución desinfectante (ver figura F).
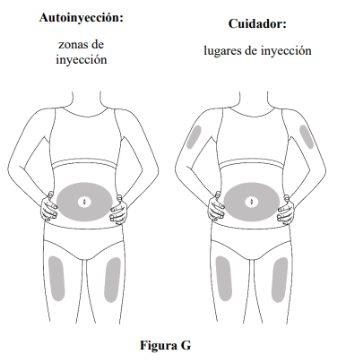
Paso 5. Seleccione el lugar de la administración SC
- Inyecte en el muslo o en el abdomen, pero manténgase a 2 cm del ombligo (ver figura G).
- Si otra persona (cuidador) le administra la inyección, también puede usar la parte superior del brazo. Nointente inyectarse usted mismo en la parte superior del brazo.
- Cambie (es decir, rote) el lugar con cada inyección. No inyecteen la misma zona varias veces si la piel está dañada.
- Noinyecte en el ombligo, lunares, cicatrices o moretones, o en áreas donde la piel esté sensible, roja, dura o lesionada.
Paso 6. Preparación del lugar de inyección
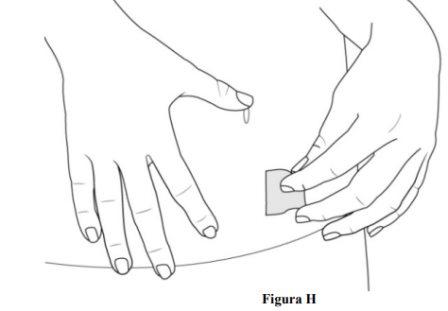
- Limpie el lugar de inyección con una toallita con alcohol (ver figura H).
- Deje que la piel se seque al aire.
- Novuelva a tocar esta zona antes de la administración.
- Noabanique ni sople sobre el área de la piel que ha limpiado.
Inyección del medicamento con la pluma precargada
Complete la inyección sin pausas. Lea todos los pasos antes de comenzar. No retire el capuchón transparente hasta el momento de la inyección. |
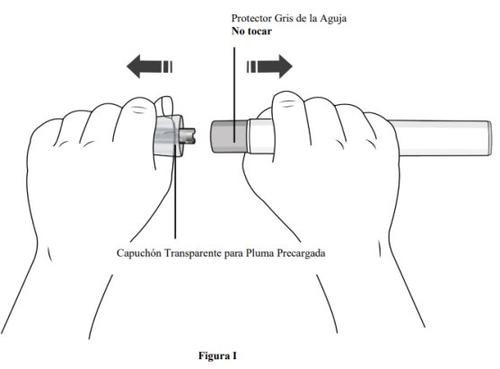
Paso 7. Retire el capuchón transparente de la pluma precargada y deséchelo
- Sujete la pluma precargada con una mano y retireel capuchón transparente de la pluma precargada tirando de élcon la otra mano.
- Nogire el capuchón transparente (ver figura I). Si no puede quitar el capuchón transparente, pida ayuda a un médico o póngase en contacto con su profesional sanitario.
- El capuchón transparente tiene una parte metálica en su interior, esto es normal.
- Novuelva a colocar el capuchón transparentedespués de retirarlo, ya que podría iniciar la inyección y provocar lesiones.
- Deseche el capuchón de la aguja en un contenedor para objetos punzantes o resistente a perforaciones.
Importante:
|
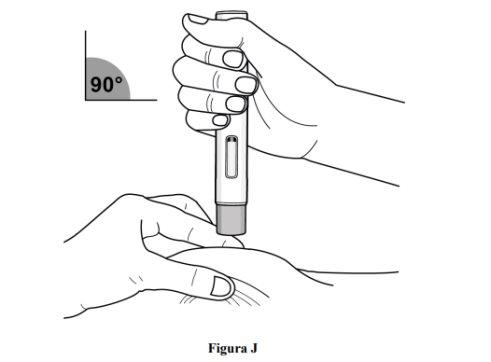
Paso 8. Pellizque la piel y coloque la pluma precargada en el lugar de inyección
Inmediatamente después de retirar el capuchón transparente de la pluma recargada, complete los siguientes pasos sin detenerse:
- Pellizque suavemente la zona de piel limpia alrededor del lugar de inyección y sostenga la zona firmemente hasta que se complete la inyección (ver figura J).
- Coloque la pluma precargada en un ángulo de 90° sobre el lugar de inyección limpio (ver figura J).
- Asegúrese de poder ver la ventana de visualización.
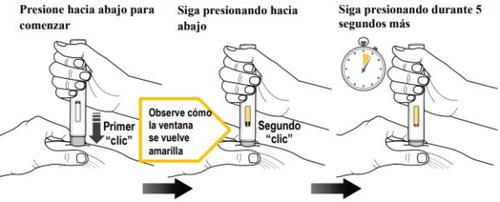
Paso 9. Inyección del Medicamento(ver figura K)
| Debe leer todo el contenido del paso 9 antes de la administración. La inyección puede tardar hasta 15 segundos. Para asegurarse de recibir una dosis completa, debe mantener la pluma precargada firmemente presionada contra la piel pellizcada hasta que:
y
|
Presione el protector gris de la aguja firmemente contra la piel pellizcada para iniciar la administración y siga presionando hasta completar todos los pasos siguientes. | ||
| ||
Presione hacia abajo para iniciarla inyección y espere a escuchar un primer “clic”. | Mantenga presionadala pluma precargada hacia abajo y observe la ventana de visualización. | Mantenga presionadala pluma precargada hacia abajodurante 5 segundos más para asegurarse de obtener la dosis completa. |
|
amarilla y
| |
Siga presionandola pluma precargada hacia abajo. | Siga presionandola pluma precargada hacia abajo. |
Figura K
- Noretire la pluma precargada hasta que el émbolo amarillo deje de moverse y llene completamente la ventana de visualización, y hayan pasado 5 segundos después del segundo “clic”.
- No retire, incline ni gire la pluma precargada durante la inyección.
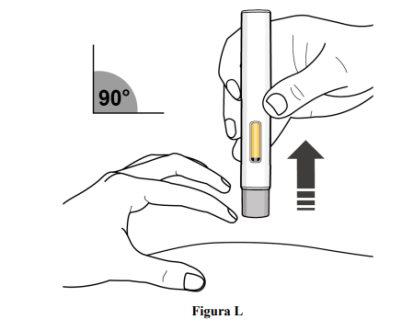
Paso 10. Deje de pellizcar y retire la pluma precargada
- Deje de pellizcar la piel y retire la pluma precargada de la piel con un ángulo de 90º (ver figura L).
- A medida que la pluma precargada se levanta de la piel, el protector gris de la aguja volverá a la posición original (antes del uso) y se bloqueará en su posición para cubrir la aguja.
Importante: Si cree que no ha recibido la dosis completa, póngase en contacto de inmediato con su prestador de asistencia sanitaria.
- Si hay un poco de sangrado en el lugar de inyección, puede presionar con una torunda de algodón o una gasa sobre la zona de la inyección.
- Nofrote el lugar de inyección.
- Si es necesario, puede cubrir el punto de administración con una pequeña venda adhesiva.
Residuo
Paso 11. Eliminación de la pluma precargada
- Noreutilice la jeringa precargada.
- Después de administrarse la dosis, coloque la pluma precargada en un contenedor para objetos punzantes o en un contenedor resistente a perforaciones cerrado (ver figura M).
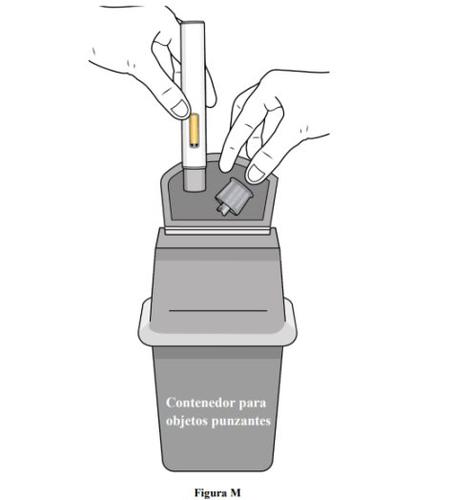

- Si no tiene un recipiente para objetos punzantes o un contenedor cerrado resistente a perforaciones, puede utilizar un contenedor doméstico que sea:
- Fabricado en plástico resistente.
- Se puede cerrar con una tapa hermética y resistente a perforaciones, sin que las partes afiladas puedan salir.
- Estable en posición vertical durante el uso
- Resistente a fugas
- Debidamente etiquetado para advertir sobre residuos peligrosos dentro del contenedor
- Cuando su contenedor de objetos punzantes esté casi lleno, deberá seguir las pautas locales para saber cuál es la forma correcta de desecharlo. Pregúntele a su farmacéutico o profesional sanitario para obtener más información sobre cómo eliminar su contenedor de objetos punzantes.
- Noelimine el contenedor de objetos punzantes usados con la basura doméstica a menos que las pautas locales lo permitan.
- Norecicle su contenedor de objetos punzantes usados.
Paso 12. Seguimiento del tratamiento
- Si su médico lo requiere, registre la administración en un diario para ayudar a realizar un seguimiento de su medicamento.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ANDEMBRY 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 1500 UIPrincipio activo: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequiere recetaForma farmacéutica: INYECTABLE, 2000 IUPrincipio activo: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequiere recetaForma farmacéutica: INYECTABLE, 3000 IUPrincipio activo: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequiere receta
Médicos online para ANDEMBRY 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ANDEMBRY 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes