SPRAVATO 28 mg NASAL SPRAY SOLUTION


How to use SPRAVATO 28 mg NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Spravato 28 mg Nasal Spray Solution
esketaamine
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Spravato and what is it used for
- What you need to know before you use Spravato
- How to use Spravato
- Possible side effects
- Storage of Spravato
- Contents of the pack and other information
1. What is Spravato and what is it used for
What is Spravato
Spravato contains the active substance esketamine. This belongs to a group of medicines called antidepressants and has been given to you to treat your depression.
What Spravato is used for
Spravato is used in adults to reduce symptoms of depression, such as feeling sad, anxious or useless, difficulty sleeping, changes in appetite, loss of interest in favorite activities, feeling slowed down. It is given together with another antidepressant, if you have tried at least two other antidepressants but they have not helped.
Spravato is also used in adults to quickly reduce symptoms of depression in a situation that requires immediate treatment (also known as a psychiatric emergency).
2. What you need to know before you use Spravato
Do not use Spravato
- if you are allergic to esketamine, a similar medicine called ketamine used for anesthesia or any of the other ingredients of this medicine (listed in section 6).
- if you have ever had certain disorders such as:
- an aneurysm (a weak spot in the wall of a blood vessel where it widens or bulges)
- brain hemorrhage
- if you have recently had a heart attack (in the last 6 weeks)
The reason is that Spravato may cause a temporary increase in blood pressure that can cause serious complications in these conditions.
Do not use Spravato if you are in any of the above circumstances. If you are not sure, talk to your doctor before using Spravato; your doctor will decide if you can or cannot use this medicine.
Warnings and precautions
Talk to your doctor before starting to use Spravato if:
- you have a heart problem that is not well controlled, such as poor blood flow in the blood vessels of the heart, often with chest pain (such as angina), high blood pressure, heart valve problems or heart failure
- you have ever had problems with blood supply to the brain (such as a stroke)
- you have ever had problems with drug abuse (prescription or illegal drugs)
- you have ever had a disease called psychosis, in which you believe things that are not true (delusions) or see, feel or hear things that do not exist (hallucinations)
- you have ever had a condition called bipolar disorder, or symptoms of mania (in which you are overactive or overexcited)
- you have ever had an overactive thyroid that has not been properly treated (hyperthyroidism)
- you have ever had lung problems that cause difficulty breathing (lung failure), including chronic obstructive pulmonary disease (COPD)
- you have sleep apnea and are severely overweight
- you have ever had slow or fast heartbeats that cause difficulty breathing, palpitations or chest discomfort, feeling dizzy or fainting
- you have had a severe head injury or serious problems that affected your brain, especially when there is increased pressure in the brain
- you have severe liver problems.
If you meet any of the above conditions (or if you are not sure), talk to your doctor before starting to use Spravato. Your doctor will decide if you should use this medicine.
Worsening of depression
Tell your doctor or go immediately to the nearest hospital if at any time you think about harming yourself or committing suicide.
It may be helpful to talk to a family member or close friend if you are depressed and ask them if they think your depression is getting worse or if they are concerned about your behavior. You can ask them to read this leaflet.
Blood pressure
Spravato may increase your blood pressure for about 1 to 2 hours after using it, so your blood pressure will be measured before you start using Spravato and after using it.
If your blood pressure is high before using this medicine, your doctor will decide if you should start using it or wait until your blood pressure is lower. If your blood pressure increases after using this medicine and remains high for more than a few hours, you may need to have some additional tests.
This medicine may cause a temporary increase in blood pressure after administering a dose. Your blood pressure will be measured before and after using this medicine. Tell your doctor immediately if you have chest pain, difficulty breathing, sudden severe headache, changes in vision or seizures (fits) after using this medicine.
Tell your doctor if you experience any of the following symptoms while using Spravato
- difficulty with your attention, judgment and thinking (see also “Driving and using machines” and “Possible side effects”). During and after each use of this medicine, your doctor will check your condition and decide how long you should be monitored.
- drowsiness (sedation), fainting, dizziness, feeling of spinning, anxiety or feeling disconnected from yourself, your thoughts, feelings, space and time (dissociation), difficulty breathing (respiratory depression). Tell your doctor immediately if you feel that you cannot stay awake or feel that you are going to faint.
- difficulty urinating or blood in your urine; these may be signs of bladder problems. These can occur with high doses of a similar medicine (called ketamine) used for a long period.
Tell your doctor if you experience any of the above symptoms while using Spravato.
Elderly patients (> 65 years)
If you are older (> 65 years), you will be closely monitored, as you may have a higher risk of falling when you start moving after treatment.
Children and adolescents
Do not give this medicine to children or adolescents under 18 years of age. This is because Spravato has not been studied in treatment-resistant depression in this age group.
Other medicines and Spravato
Tell your doctor if you are taking, have recently taken or might take any other medicines.
Using Spravato with certain medicines may cause side effects. Tell your doctor especially if you are taking:
- Medicines used to treat nervous disorders or severe pain (e.g. benzodiazepines, opioids), or medicines or drinks that contain alcohol
- Stimulants such as those used for conditions like narcolepsy or medicines for ADHD (e.g. amphetamines, methylphenidate, modafinil, armodafinil)
- Medicines that can increase your blood pressure, such as thyroid hormones, asthma medicines like xanthine derivatives, medicines for postpartum hemorrhage (ergometrine) and heart medicines like vasopressin.
- Medicines for depression or Parkinson's disease called monoamine oxidase inhibitors (MAOIs) (e.g. tranylcypromine, selegiline, phenelzine).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
Contraceptive measures
If you can become pregnant, you should use contraceptive measures during treatment. Talk to your doctor about suitable contraceptive methods.
Pregnancy
Do not use Spravato if you are pregnant.
If you become pregnant while being treated with Spravato, talk to your doctor immediately to decide whether to stop treatment and explore other treatment options.
Breastfeeding
Do not use Spravato if you are breastfeeding. Talk to your doctor before using Spravato if you are breastfeeding. Your doctor will discuss with you whether you should stop breastfeeding or stop using this medicine. Your doctor will consider the benefit of breastfeeding for you and your baby and the benefit of treatment for you.
Driving and using machines
Spravato may make you feel drowsy, dizzy and suffer other side effects that can temporarily affect your ability to drive vehicles or use machines and perform activities that require you to be fully alert. After receiving treatment with this medicine, do not perform these activities until the day after a good night's sleep.
3. How to use Spravato
Follow exactly the instructions for administering this medicine given by your doctor.
In case of doubt, consult your doctor.
You will use the Spravato nasal spray yourself under the supervision of a doctor, or another healthcare professional in a healthcare setting, such as the doctor's office or clinic.
Your doctor or another healthcare professional will teach you how to use the nasal spray (see also Instructions for use).
Recommended dose
Your doctor will decide if you need 1, 2 or 3 nasal sprays and how often you should visit the doctor's office or clinic to receive the medicine.
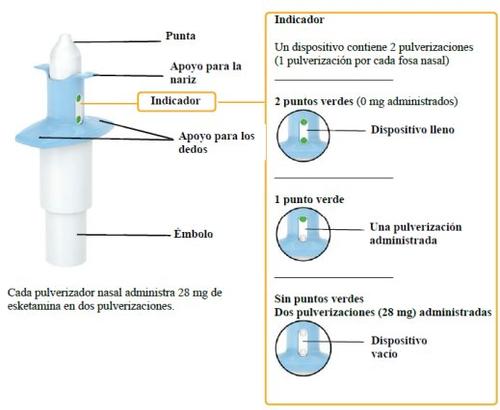
- One nasal spray gives two sprays (one per nostril)
- Spravato is used twice a week for the first 4 weeks
If your treatment is continued:
- Spravato is usually used once a week for the next 4 weeks
- After that, Spravato is usually used once a week or once every 2 weeks.
During and after each use of this medicine, your doctor will check your condition and decide how long you should be monitored.
Food and drink
Some patients using Spravato may experience nausea or vomiting. You should avoid eating for 2 hours before treatment and not drink liquids for 30 minutes before using this medicine.
Nasal sprays
If you need medicines with steroids or decongestants in the form of nasal sprays, avoid using them during the hour before treatment with Spravato.
If you use more Spravato than you should
You will use this medicine under the supervision of a doctor in the doctor's office or clinic. Therefore, it is unlikely that you will use too much.
If you use too much Spravato, you are more likely to experience side effects (see “Possible side effects”).
If you stop using Spravato
It is important that you make sure to attend scheduled appointments so that this medicine is effective for you.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor if you notice any of the following side effects.
Very common(may affect more than 1 in 10 people)
- feeling disconnected from yourself, your thoughts, feelings and the things around you
- feeling dizzy
- headache
- drowsiness
- altered sense of taste
- decreased sensation or sensitivity, including around the mouth area
- feeling that everything is spinning (“vertigo”)
- vomiting
- nausea
- increased blood pressure
Common(may affect up to 1 in 10 people)
- feeling anxious
- feeling extremely happy (“euphoria”)
- feeling confused
- feeling disconnected from reality
- feeling irritable
- seeing, feeling, hearing or smelling things that are not there (hallucinations)
- feeling agitated
- your eyes, ears or sense of touch are confused or deceived in some way (something is not what it seems)
- panic attacks
- change in time perception
- unusual sensation in the mouth (such as tingling or numbness)
- muscle tremors
- problems thinking
- feeling very drowsy with little energy
- difficulty speaking
- difficulty concentrating
- blurred vision
- persistent ringing in the ears (tinnitus)
- increased sensitivity to noise or sounds
- fast heartbeat
- high blood pressure
- nasal discomfort
- throat irritation
- sore throat
- dry nose including dry crusts in the nose
- itching in the nose
- decreased sensations or sensitivity in the mouth
- dry mouth
- excessive sweating
- frequent need to urinate
- difficulty urinating
- urgent need to urinate
- unusual sensation
- feeling drunk
- feeling weak
- crying
- feeling of changed body temperature
Uncommon(may affect up to 1 in 100 people)
- slowing of thoughts, speech and physical movements
- emotional stress
- feeling restless or tense
- rapid eye movements that cannot be controlled
- hyperactivity
- increased saliva
- cold sweats
- problems walking
Rare(may affect up to 1 in 1,000 people)
- difficulty breathing (respiratory depression)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Spravato
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label.
The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Spravato Composition
The active ingredient is esketamine.
Each nasal spray device contains esketamine hydrochloride equivalent to 28 mg of esketamine.
The other ingredients are:
Citric acid monohydrate
Disodium edetate
Sodium hydroxide (for pH adjustment)
Water for injectable preparations
Product Appearance and Package Contents
Spravato is a nasal spray solution. This medication is a clear, colorless solution presented in a single-use nasal spray device.
Spravato is available in packages containing 1, 2, 3, or 6 nasal spray devices and as a multiple package containing 24 (8 packages of 3) nasal spray devices.
Each nasal spray device is individually packaged in a sealed blister.
Only some package sizes may be marketed.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Janssen-Cilag NV Tel: +32 14 64 94 11 | Lithuania UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
Bulgaria “Johnson & Johnson” EAD Tel: +359 2 489 94 00 | Luxembourg Janssen-Cilag NV Tel: +32 14 64 94 11 |
Czech Republic Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Hungary Janssen-Cilag Kft. Tel: +36 1 884 2858 |
Denmark Janssen-Cilag A/S Tel: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Germany Janssen-Cilag GmbH Tel: +49 2137 955 955 | Netherlands Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Estonia UAB "JOHNSON & JOHNSON" Estonian branch Tel: +372 617 7410 | Norway Janssen-Cilag AS Tel: +47 24 12 65 00 |
Greece Janssen-Cilag Φαρμακευτική Α.Ε.Β.Ε. Tel: +30 210 80 90 000 | Austria Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
Spain Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Poland Janssen-Cilag Polska Sp. z o.o. Tel: +48 22 237 60 00 |
France Janssen-Cilag Tel: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Croatia Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | Romania Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenia Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Iceland Janssen-Cilag AB c/o Vistor hf. Tel: +354 535 7000 | Slovakia Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italy Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Finland Janssen-Cilag Oy Tel: +358 207 531 300 |
Cyprus Βαρνάβας Χατζηπαναγής Λτδ Tel: +357 22 207 700 | Sweden Janssen-Cilag AB Tel: +46 8 626 50 00 |
Latvia UAB "JOHNSON & JOHNSON" Latvian branch Tel: +371 678 93561 | United Kingdom(Northern Ireland) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Date of Last Revision of this Leaflet:{MM/YYYY} {Month YYYY}.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
The following information is intended for healthcare professionals only:
Instructions for Use
SPRAVATO
(esketamine)
Nasal Spray Device

28 mg per device
Each nasal spray device administers 28 mg of esketamine in two sprays.
Important
This device is intended for patient administration, under the supervision of a healthcare professional. Read these Instructions for Use carefully before training and supervising the patient.
Need Help?
If you need further assistance or would like to share your opinion, see the contact information of the local representative of the marketing authorization holder in the package leaflet.
Nasal Spray Device

Only before the first device:
| Instruct the patient to blow their nose only before the first device. |
| Confirm the required number of devices. |


- Check the expiration date ('CAD'). If it has expired, obtain a new device.
- Open the blister pack and remove the device.
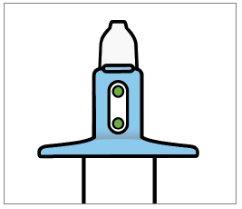
- Do not prime (load) the device.This will result in loss of medication.
- Check that the indicator shows 2 green dots. If not, discard the device and obtain a new one.
- Hand the device to the patient.

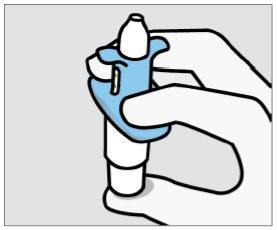
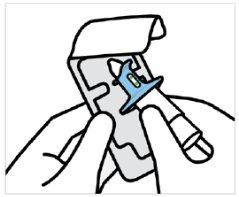
Instruct the patient to:
- Hold the device as shown, with the thumb gently resting on the plunger.
- Do notpress the plunger.
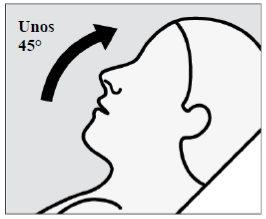
Instruct the patient to:
- Tilt the head back at an angle of about 45 degreesduring administration to keep the medication inside the nose.

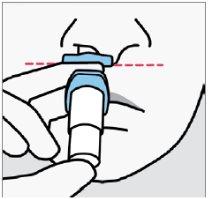
Instruct the patient to:
- Insert the tip directly into the first nostril.
- The nasal support should touch the skin between the nostrils.
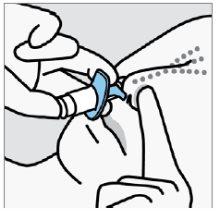
Instruct the patient to:
- Block the opposite nostril.
- Breathe in through the nosewhile pushing the plunger up to the stop.
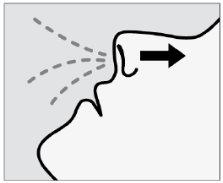
Instruct the patient to:
- Breathe in slowlyafter the spray to keep the medication inside the nose.
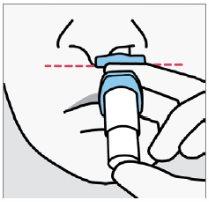
Instruct the patient to:
- Switch hands to insert the tipinto the second nostril.
- Repeat step 4 to administer the second spray.

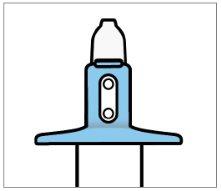
- Retrieve the device from the patient.
- Check that the indicator shows no green dots.If you see a green dot, have the patient repeat the spray in the second nostril.
- Check the indicator again to confirm that the device is empty.
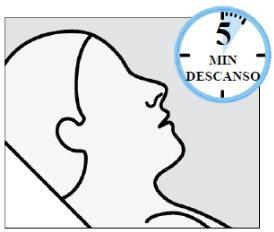
Instruct the patient to:
- Rest in a comfortable position (preferably semi-reclined) for 5 minutes after each device.
- If liquid drips, wipe the nose with a tissue paper.
 Do not blow your nose.
Do not blow your nose.

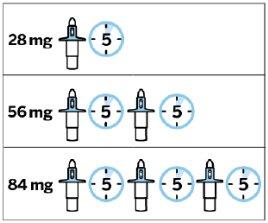
- Repeat steps 2-5if more than one device is needed.


Reviewed: {Month YYYY}
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SPRAVATO 28 mg NASAL SPRAY SOLUTIONDosage form: TABLET, 15 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 30 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 25 mgActive substance: agomelatineManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for SPRAVATO 28 mg NASAL SPRAY SOLUTION
Discuss questions about SPRAVATO 28 mg NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions