
PRIMPERAN 1mg /ml SOLUCION ORAL

Cómo usar PRIMPERAN 1mg /ml SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Primperan 1 mg/ml solución oral
Metoclopramida hidrocloruro
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Primperan y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Primperan
- Cómo tomar Primperan
- Posibles efectos adversos
- Conservación de Primperan
- Contenido del envase e información adicional
1. Qué es Primperan y para qué se utiliza
Primperan es un antiemético. Contiene un medicamento denominado “metoclopramida”. Actúa en una zona del cerebro que previene las náuseas o los vómitos.
Población adulta
Primperan se usa en adultos:
- para prevenir las náuseas y vómitos retardados que pueden aparecer después de la quimioterapia
- para prevenir las náuseas y vómitos provocados por la radioterapia
- para tratar las náuseas y vómitos, incluyendo las náuseas y vómitos que pueden aparecer con una migraña
Se puede tomar metoclopramida en combinación con analgésicos orales en el caso de migraña para que los analgésicos sean más efectivos.
Población pediátrica
Primperan está indicado en niños (1-18 años de edad) si otros tratamientos no funcionan o no se pueden utilizar para prevenir las náuseas y vómitos retardados que pueden aparecer después de la quimioterapia.
2. Qué necesita saber antes de empezar a tomar Primperan
No tome Primperan
- si es alérgico a metoclopramida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si padece hemorragia, obstrucción o perforación en el estómago o intestino
- si tiene o podría tener un tumor raro de la glándula adrenal, que está cerca del riñón (feocromocitoma)
- si ha sufrido alguna vez espasmos involuntarios de los músculos (discinesia tardía), cuando ha sido tratado con un medicamento
- si tiene epilepsia
- si tiene la enfermedad de Parkinson
- si está tomando levodopa (medicamento para la enfermedad de Parkinson) o agonistas dopaminérgicos (ver debajo “Otros medicamentos y Primperan”)
- si ha tenido alguna vez niveles anormales de pigmentos de la sangre (metahemoglobinemia) o deficiencia de NADH citocromo b5 reductasa.
No administre Primperan a niños menores de 1 año (ver debajo “Niños y adolescentes”).
No tome Primperan si alguno de los casos de arriba le aplica. Si no está seguro, consulte a su médico, farmacéutico o enfermero antes de tomar Primperan.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de tomar Primperan si:
- tiene antecedentes de latidos de corazón anormales (prolongación del intervalo QT) o cualquier otro problema del corazón
- tiene problemas con los niveles de sales en su sangre, como potasio, sodio y magnesio
- está usando otros medicamentos conocidos por afectar a la forma de latir de su corazón
- tiene algún problema neurológico (cerebro)
- tiene problemas en el hígado o en los riñones. Se puede reducir la dosis (ver sección 3).
Su médico puede realizar análisis de sangre para controlar sus niveles de pigmentos de la sangre. En casos de niveles anormales (metahemoglobinemia) se debe interrumpir el tratamiento de forma inmediata y permanentemente.
Debe esperar al menos 6 horas entre cada dosis de metoclopramida, incluso en el caso de vómito o rechazo de la dosis, para evitar la sobredosis.
No exceder 3 meses de tratamiento por el riesgo de espasmos musculares involuntarios.
Niños y adolescentes
Pueden aparecer movimientos incontrolables (trastornos extrapiramidales) en niños y adultos jóvenes. Este medicamento no se debe utilizar en niños menores de 1 año debido al elevado riesgo de movimientos incontrolables (ver arriba “No tome Primperan”).
Otros medicamentos y Primperan
Comunique a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento. Esto se debe a que algunos medicamentos pueden afectar a la forma de actuar de Primperan o Primperan puede afectar a la forma de actuar de otros medicamentos. Estos medicamentos incluyen los siguientes:
- levodopa u otros medicamentos utilizados para tratar la enfermedad de Parkinson (ver arriba “No tome Primperan”)
- anticolinérgicos (medicamentos utilizados para aliviar espasmos o calambres del estómago)
- derivados de la morfina (medicamentos utilizados para tratar el dolor intenso)
- medicamentos sedantes
- cualquier medicamento utilizado para tratar problemas de salud mental
- digoxina (medicamento utilizado para tratar la insuficiencia del corazón)
- ciclosporina (medicamento utilizado para tratar algunos problemas del sistema inmunológico)
- mivacurio y suxametonio (medicamentos utilizados para relajar los músculos)
- fluoxetina y paroxetina (medicamentos utilizados para tratar la depresión)
- rifampicina (medicamento utilizado para tratar la tuberculosis u otras infecciones), puede reducir la cantidad de metoclopramida en la sangre si se administra al mismo tiempo.
Uso de Primperan con alcohol
No se debe consumir alcohol durante el tratamiento con metoclopramida porque aumenta el efecto sedante de Primperan.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Si es necesario, se puede tomar Primperan durante el embarazo. Su médico decidirá si se debe o no administrar este medicamento.
No está recomendado Primperan si está en periodo de lactancia porque metoclopramida pasa a la leche materna y puede afectar a su bebé.
Conducción y uso de máquinas
Después de tomar Primperan se puede sentir somnoliento, mareado o tener movimientos incontrolables de tics, sacudidas o de torsión y tono de los músculos no usual que cause distorsión de su cuerpo. Esto puede afectar a su visión y también interferir en su capacidad para conducir y utilizar máquinas.
Primperan 1 mg/ml solución oral contiene parahidroxibenzoato de metilo y parahidroxibenzoato de propilo
Puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo y parahidroxibenzoato de propilo.
Primperan 1 mg/ml solución oral contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por 1 ml de solución oral; esto es, esencialmente “exento de sodio”.
Primperan 1 mg/ml solución oral contiene alcohol
Este medicamento contiene 4,9 mg de alcohol (etanol) en cada ml de solución oral que equivale a 0,49 % (p/v). La cantidad en un ml de solución oral de este medicamento es equivalente a menos de 0,12 ml de cerveza o 0,05 ml de vino.
La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible.
3. Cómo tomar Primperan
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Todas las indicaciones (pacientes adultos)
Se recomienda una dosis única de 10 mg, que se puede repetir hasta tres veces al día.
La dosis máxima diaria recomendada es de 30 mg o 0,5 mg/kg de peso corporal.
La duración máxima del tratamiento es de 5 días.
Para prevenir las náuseas y vómitos retardados que pueden aparecer después de la quimioterapia (niños de 1-18 años de edad)
La dosis recomendada es de0,1 a0,15 mg/kg de peso corporal, que se puede repetir hasta tres veces al día, tomada por la boca (vía oral).
La dosis máxima en 24 horas es 0,5 mg/kg de peso corporal.
Tabla de dosis
Edad | Peso corporal | Dosis | Frecuencia |
1-3 años | 10-14 kg | 1 mg | Hasta 3 veces al día |
3-5 años | 15-19 kg | 2 mg | Hasta 3 veces al día |
5-9 años | 20-29 kg | 2,5 mg | Hasta 3 veces al día |
9-18 años | 30-60 kg | 5 mg | Hasta 3 veces al día |
15-18 años | Más 60 kg | 10 mg | Hasta 3 veces al día |
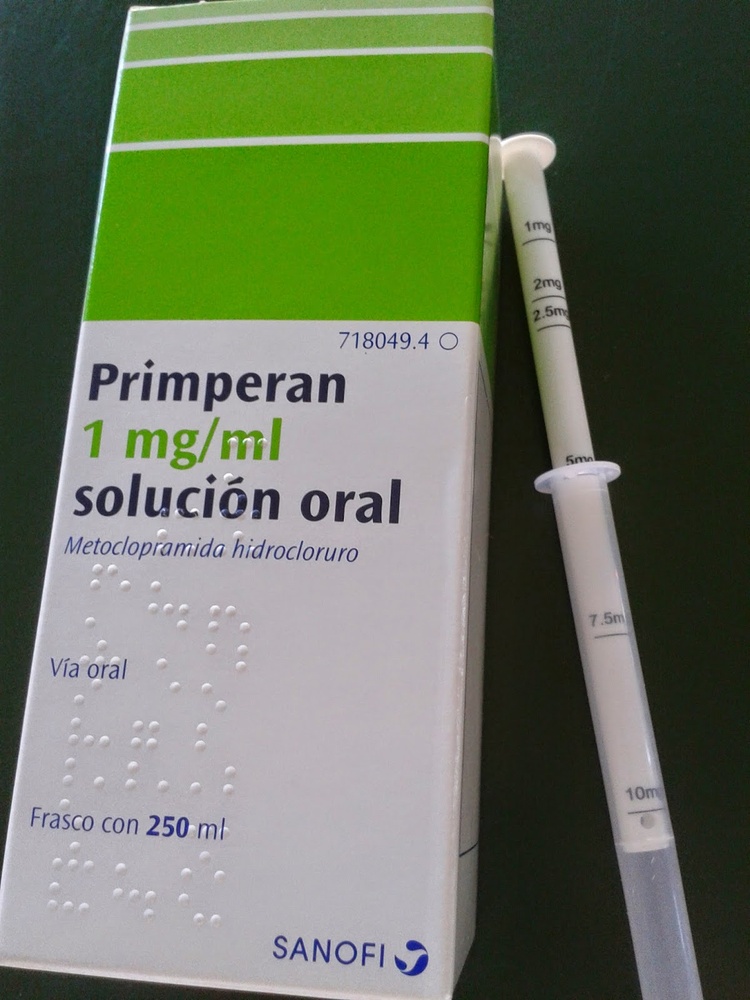
Utilice la jeringa oral de medición que se incluye en el envase de la solución oral para poder administrar la dosis adecuada de metoclopramida.
La jeringa oral de medición está graduada en mg. La correspondencia con el peso corporal se detalla en la tabla de dosis. La dosis se consigue tirando del émbolo hasta la graduación en mg correspondiente.
El uso de la jeringa oral de medición está restringido a la administración de esta solución.
Se debe enjuagar la jeringa oral de medición después de cada uso.
No se debe sumergir la jeringa oral de medición en la botella.
No debe tomar este medicamento durante más de 5 días para prevenir las náuseas y vómitos retardados que pueden aparecer después de la quimioterapia.
Forma de administración
Debe esperar al menos 6 horas entre cada dosis de metoclopramida, incluso en el caso de vómito o rechazo de la dosis, para evitar la sobredosis.
Poblaciones especiales
Población de edad avanzada
Puede ser necesario reducir la dosis dependiendo de los problemas de los riñones, problemas en el hígado y de los problemas de salud en general.
Adultos con problemas renales
Informe a su médico si tiene problemas en los riñones. Se debe reducir la dosis si tiene problemas renales de moderados a graves.
Adultos con problemas hepáticos
Informe a su médico si tiene problemas en el hígado. Se debe reducir la dosis si tiene problemas hepáticos graves.
Niños y adolescentes
No se debe usar metoclopramida en niños de menos de 1 año (ver sección 2).
Si toma más Primperan del que debe
Contacte inmediatamente con su médico o farmacéutico. Puede experimentar movimientos incontrolables (trastornos extrapiramidales), sentir somnolencia, tener algunos problemas de consciencia, estar confuso, tener alucinaciones y problemas en el corazón. Su médico puede recetarle un tratamiento para estos efectos si fuera necesario.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o a su farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Primperan
No tome una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, enfermero o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuencia no conocida(frecuencia que no se puede estimar a partir de los datos disponibles)
- reacciones alérgicas (como anafilaxia, angioedema y urticaria).
Los síntomas pueden incluir erupción, picor, dificultad para respirar, falta de aire, hinchazón de la cara, labios, garganta o lengua, frío, piel húmeda, palpitaciones, mareos, debilidad o desmayo. Contacte inmediatamente con su médico u otro profesional sanitario o vaya al servicio de urgencias del hospital más cercano de inmediato.
Interrumpa el tratamiento e informe inmediatamente a su médico, farmacéutico o enfermero si experimenta uno de los siguientes signos mientras esté tomando este medicamento:
- movimientos incontrolables (que afectan a menudo a la cabeza y al cuello). Estos pueden aparecer en niños y adultos jóvenes y particularmente cuando se usan dosis altas. Estos signos aparecen normalmente al principio del tratamiento e incluso se pueden presentar después de una única administración. Estos movimientos cesarán cuando se traten adecuadamente.
- fiebre alta, presión arterial alta, convulsiones, sudoración, producción de saliva. Estos pueden ser signos de una condición denominada síndrome neuroléptico maligno.
- picor y erupciones cutáneas, inflamación de la cara, labios o garganta, dificultad para respirar. Estos pueden ser signos de una reacción alérgica, que puede ser grave.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- sentirse somnoliento.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- depresión
- movimientos incontrolables como tics, sacudidas, movimientos de torsión o contracturas en los músculos (agarrotamiento, rigidez)
- síntomas similares a la enfermedad de Parkinson (rigidez, temblor)
- sentirse inquieto
- disminución de la presión arterial (particularmente con la administración intravenosa)
- diarrea
- sentirse débil.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- niveles elevados en la sangre de una hormona denominada prolactina que puede producir: producción de leche en los hombres y mujeres que no están en periodo de lactancia
- periodos irregulares
- alucinaciones
- nivel de consciencia disminuido
- ritmo lento del corazón (particularmente con la administración intravenosa)
- alergia
- alteraciones visuales y desviación involuntaria del globo ocular.
Raros(pueden afectar hasta 1 de cada 1.000 personas)
- estado de confusión
- convulsiones (especialmente en pacientes con epilepsia).
Frecuencia no conocida(frecuencia que no se puede estimar a partir de los datos disponibles)
- niveles anormales de pigmentos de la sangre: que pueden cambiar el color de su piel
- desarrollo anormal de las mamas (ginecomastia)
- espasmos musculares involuntarios después del uso prolongado, particularmente en pacientes de edad avanzada
- fiebre alta, presión arterial alta, convulsiones, sudoración, producción de saliva. Estos pueden ser signos de una enfermedad denominada síndrome neuroléptico maligno
- cambios en los latidos del corazón, que pueden verse en un ECG (electrocardiograma)
- paro cardiaco (particularmente con la administración intravenosa)
- shock (descenso intenso de la presión del corazón) (particularmente con la administración intravenosa)
- desmayo (particularmente con la administración intravenosa)
- presión arterial muy elevada
- ideas suicidas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Primperan
Mantener este medicamento fuera de la vista y del alcance de los niños.
No se precisan condiciones especiales de conservación.
No utilice Primperan después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Primperan 1 mg/ml solución oral
- El principio activo es metoclopramida hidrocloruro. Cada ml de solución oral contiene 1 mg de metoclopramida hidrocloruro.
- Los demás componentes son: hidroxietilcelulosa, ciclamato de sodio, sacarina de sodio, parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216), ácido cítrico monohidrato, esencia de albaricoque-naranja (contiene etanol), agua purificada.
Aspecto del producto y contenido del envase
Es un líquido transparente viscoso, incoloro a ligeramente ámbar y con olor aromático a naranja y albaricoque.
Esta solución oral está envasada en un frasco de 200 ml con tapón a prueba de niños y se incluye una jeringa oral de medición.
Otras presentaciones:
Primperan 10 mg comprimidos: Envases con 30 y 60 comprimidos
Primperan 10 mg/2 ml solución inyectable: Envase con 12 ampollas de 2 ml
Titular de la autorización de comercialización
sanofi-aventis, S.A.
C/ Rosselló i Porcel, 21
08016 Barcelona
España
Responsable de la fabricación
- Nattermann & Cie. GmbH
Nattermannallee, 1
50829 Cologne
Alemania
o
Unither Liquid Manufacturing
Entrée 1, 3 Allée de la Neste
Zone Industrielle D’En Sigal
31770 Colomiers
Francia
Fecha de la última revisión de este prospecto: Septiembre 2023
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia2 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PRIMPERAN 1mg /ml SOLUCION ORALForma farmacéutica: COMPRIMIDO, 10 MGPrincipio activo: metoclopramideFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 5 mg/mlPrincipio activo: metoclopramideFabricante: Laboratorios Basi Industria Farmaceutica S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 1 mg/mlPrincipio activo: metoclopramideFabricante: Kern Pharma S.L.Requiere receta
Médicos online para PRIMPERAN 1mg /ml SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PRIMPERAN 1mg /ml SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















