
OXIGENO MEDICINAL GAS AIR LIQUIDE 99,5% V/V 300 BAR GAS COMPRIMIDO MEDICINAL


Cómo usar OXIGENO MEDICINAL GAS AIR LIQUIDE 99,5% V/V 300 BAR GAS COMPRIMIDO MEDICINAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar y para qué se utiliza
- Qué necesita saber antes de empezar a usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
- Cómo usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
- Posibles efectos adversos
- Conservación de Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar gas comprimido medicinal
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar y para qué se utiliza
- Qué necesita saber antes de empezar a usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
- Cómo usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
- Posibles efectos adversos
- Conservación de Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
- Contenido del envase e información adicional
1. Qué es Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar y para qué se utiliza
Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar, es un gas para inhalación que se envasa en bala de gas a 300 bares de presión a 15ºC.
El oxígeno es un elemento esencial para el organismo. El tratamiento con oxígeno está indicado en los siguientes casos:
- Corrección de la falta de oxígeno de distintos orígenes que precisan la administración de oxígeno a presión normal o elevada.
- Alimentación de los respiradores en anestesia – reanimación.
- Administración mediante nebulizador de los medicamentos para inhalación.
- Tratamiento de las fases agudas de Cefaleas tipo Cluster (Cluster Headache o Cefaleas en racimo)
2. Qué necesita saber antes de empezar a usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
No useOxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar:
El oxígeno a una presión mayor que la presión atmosférica (Oxigenoterapia Hiperbárica) no debe utilizarse en casos de neumotórax no tratado o sin drenar. Un neumotórax se debe a la acumulación de aire en la cavidad torácica entre las dos membranas pulmonares. Si alguna vez ha tenido un neumotórax, informe a su médico.
Advertencias y precauciones
Consulte a su médico, o farmacéutico o enfermero antes de empezar a usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar.
Antes de iniciar la oxigenoterapia, usted debe saber lo siguiente:
- El oxígeno puede tener efectos nocivos a altas concentraciones. Esto puede causar daños pulmonares (colapso de los alvéolos, inflamación del pulmón), lo que dificultará el aporte de oxígeno a la sangre.
- Si usted tiene una enfermedad pulmonar obstructiva crónica (EPOC) grave que causa oxigenación sanguínea insuficiente, el oxígeno se suministrará a un caudal bajo. El médico adaptará el caudal adecuado de oxigenoterapia.
- Pueden ocurrir acontecimientos adversos como daño ocular en recién nacidos, tanto a término como a prematuros. Si su bebé requiere oxígeno, el médico determinará la concentración apropiada de oxígeno a administrar.
La Oxígenoterapia hiperbárica requiere precauciones en caso de:
- Enfermedad pulmonar obstructiva crónica(EPOC)
- Enfisema pulmonar: un trastorno de los pulmones debido a la pérdida de elasticidad del tejido pulmonar acompañado de una falta (grave) de aire
- Infecciones en las vías respiratorias superiores
- Asmainsuficientemente controlada
- Cirugíareciente del oído medio
- Cirugía torácica reciente
- Fiebre alta no controlada
- Antecedentes de epilepsia o convulsiones
- Temor a los espacios confinados(claustrofobia)
- Si alguna vez ha tenido un neumotórax(acumulación de aire o gas en la cavidad torácica entre las dos membranas pulmonares)
- Problemas del corazón
Recomendaciones relacionadas con el aumento del riesgo de incendio en presencia de oxígeno:
- El oxígeno es un producto oxidante y favoroce la combustión. No se debe fumar ni haber llamas abiertas (por ejemplo, luces piloto, cocinas, hornos, fogones de gas, chispas, velas...) en las salas donde se utiliza este medicamento, ya que esto aumenta el riesgo de incendio.
- No fume ni use cigarrillos electrónicos durante todo el tratamiento con oxígeno.
- No utilice tostadoras, secadores de pelo o equipos eléctricos similares durante el tratamiento con oxígeno.
- No aplique sustancias grasas (ej. aceites, cremas, ungüentos) sobre las superficies en contacto con oxígeno. Sólo productos con base acuosa deben usarse en las manos y la cara o dentro de la nariz mientras se usa oxígeno.
- El regulador de presión se debe abrir lentamente y con cuidado para evitar el riesgo de golpe de fuego.
Las quemaduras térmicas pueden ocurrir por incendios accidentales en presencia de oxígeno.
Recomendaciones a cuidadores:
- Maneje con cuidado la bala. Asegúrese de que no haya riesgo de caída de la bala y que no esté expuesta a golpes.
- Un daño en el equipo puede causar obstrucción en la salida y/o información incorrecta mostrada en el manómetro con respecto al contenido de oxígeno restante y al flujo suministrado, conduciendo a una insuficiencia o falta de administración de oxígeno.
Niños
En prematuros y bebés recién nacidos, la oxigenoterapia puede producir daño ocular (retinopatía del prematuro). El médico determinará la concentración apropiada de oxígeno que se va a administrar para asegurar el tratamiento óptimo para su bebé.
Otros medicamentos y Oxígeno Medicinal Gas Air Liquide99,5% v/v300 bar
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Si está utilizando o ha utilizado bleomicina (para tratar el cáncer), amiodarona (para tratar enfermedades del corazón), nitrofurantoína (para tratar una infección), informe a su médico antes de usar oxígeno, ya que existe la posibilidad de que se produzcan efectos tóxicos en los pulmones.
El daño pulmonar previo causado por el pesticida Paraquat puede empeorar con la administración de oxígeno. En caso de intoxicación con Paraquat, debe evitarse el suministro de oxígeno tanto como sea posible.
Embarazo y lactancia
Este medicamento se puede utilizar durante el embarazo. pero sólo si es necesario.
Este medicamento se puede utilizar durante la lactancia.
En todas las situaciones, debe informar a su médico si está embarazada o sospecha que podría estarlo.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Puede conducir y usar máquinas mientras utiliza este medicamento siempre y cuando su médico considere que está capacitado para ello.
3. Cómo usar Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
El médico determinará la dosis correcta de este medicamento y se lo administrará mediante un sistema adecuado a sus necesidades que garantizará el suministro de la cantidad correcta de oxígeno.
Si estima que la acción de este medicamento es demasiado fuerte o débil, comuníqueselo a su médico.
Si usa másOxígeno Medicinal Gas Air Liquide99,5% v/v 300 bar del que debe
Si usted ha utilizado más Oxígeno Medicinal Gas del que debiera, consulte inmediatamente a su médico, farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20.
Hay que disminuir la concentración de oxígeno inhalado y se recomienda un tratamiento de los síntomas.
En situaciones de vulnerabilidad, la administración excesiva de este medicamento puede afectar la función respiratoria y, en casos excepcionales causar efectos adversos neurológicos que pueden conducir a una pérdida de conciencia en situaciones extremas.
El uso prolongado de este medicamento en exceso puede causar dolor asociado a la respiración, tos seca e incluso dificultad respiratoria. Si estos síntomas de sobredosis aparecen, consulte a su médico o acuda al hospital más cercano.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos generalmente aparecen a altas concentraciones y después de un tratamiento prolongado:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
En recién nacidos expuestos a altas concentraciones de oxígeno: daño ocular, que puede resultar en problemas de visión.
Con tratamiento hiperbárico: dolor de oído, miopía, barotraumatismo (daño causado a los tejidos corporales u órganos por un cambio de presión).
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
Con tratamiento hiperbárico: convulsiones.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
Colapso pulmonar
Con tratamiento hiperbárico: ruptura de la membrana timpánica.
Raros (pueden afectar hasta 1 de cada 1.000 personas):
Con tratamiento hiperbárico: falta de aire, nivel anormalmente bajo de azúcar en sangre en pacientes diabéticos.
Frecuencia desconocida (no puede estimarse a partir de los datos disponibles):
Dolor relacionado con la respiración y tos seca, sequedad en las mucosas, irritación local e inflamación de la mucosa.
Con tratamiento hiperbárico: dificultad para respirar, contracción muscular involuntaria, vértigos, alteración auditiva, otitis serosa aguda, náuseas, comportamiento anormal, disminución de la visión periférica, cambios visuales, nubosidad del cristalino (catarata).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, o farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlo directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta. La fecha de caducidad es el último día del mes que se indica.
Deben seguirse todas las normas relativas a la manipulación de recipientes a presión.
En relación con el almacenamiento y el transporte debe tenerse en cuenta lo siguiente:
Almacenamiento de las balas de gas:
- Las balas de gas deben almacenarse en un local aireado o ventilado, protegido de las inclemencias del tiempo, limpio, sin materiales inflamables, reservado al almacenamiento de gases de uso médico y que pueda cerrarse con llave.
- Las balas de gas vacías y las balas de gas llenas deben almacenarse por separado.
- Las balas de gas deben protegerse del riesgo de golpes o de caídas, así como de las fuentes de calor o de ignición, de las temperaturas iguales o superiores a 50ºC y también de los materiales combustibles y de las inclemencias del tiempo.
Almacenamiento de las balas de gas en el servicio usuario y a domicilio:
- La bala de gas debe instalarse en una ubicación que permita protegerla de los riesgos de golpes y de caídas (como un soporte con cadenas de fijación), de las fuentes de calor o de ignición, de temperaturas iguales o superiores a 50ºC, de materiales combustibles y de las inclemencias del tiempo.
- Debe evitarse el almacenamiento excesivo de envases.
Transporte de las balas de gas:
- Las balas de gas deben transportarse con ayuda de material adecuado (como una carretilla provista de cadenas, barreras o anillos) para protegerlas del riesgo de golpes o caídas. Asimismo, debe prestarse una atención especial al fijar el reductor para evitar riesgos de rupturas accidentales.
- Durante el transporte en vehículos, las balas de gas deben estar sólidamente agrupadas. Es obligatoria la ventilación permanente del vehículo y fumar debe estar prohibido terminantemente.
6. Contenido del envase e información adicional
Composición de Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar
El principio activo es Oxígeno, con una concentración mayor del 99,5% v/v y comprimido a una presión de 300 bar (15ºC).
No contiene ningún excipiente.
Aspecto del producto y contenido del envase
Oxígeno Medicinal Gas Air Liquide 99,5% v/v 300 bar, es un gas para inhalación que se envasa en balas de gas a 300 bar de presión a 15ºC.
Tipo de envase | Capacidad de agua (L) (+/-5%) | Cantidad de producto gaseoso liberado a 1 atm y 15ºC (m3) (+/-5%) | Peso del producto almacenado (Kg) (+/-5%) |
B2 | 1,9-2,1 | 0,6 | 0,8 |
Tipo de envase | Material del envase | Tipo de válvula |
B2 | Composite (revestimiento interior metálico en aluminio) | VIPR* |
B2 | Composite (revestimiento interior metálico en acero) | VIPR* |
- VIPR: Válvula con regulador de presión integrado
Instrucciones de uso de la válvula:
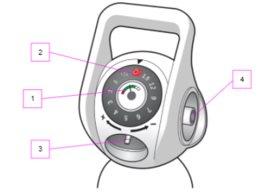
Antes de usar
- Leer el indicador de presión (1) y comprobar la cantidad de oxígeno restante
- Asegúrese de que haya flujo de gas girando el selector de flujo (2)
- Luego detenga el flujo girando el selector de flujo (2) nuevamente a “0”
Administración al paciente.
- Conecte el tubo a la salida de flujo (3)
- Gire el selector de flujo (2) en sentido horario hasta la selección de flujo deseada
- Asegúrese de que el paciente reciba oxígeno
- Después de cada uso, detenga el flujo girando el selector de flujo (2) nuevamente a "0"
Conexión de un dispositivo a la salida de presión (4) (por ejemplo, un ventilador)
Recordatorio: No utilice la salida de flujo (3) y la salida de presión (4) simultáneamente
- Primero, conecte el tubo flexible al dispositivo
- Luego, conecte el tubo flexible a la salida de presión (4)
- El oxígeno ya está disponible
- Después de su uso, se recomienda desconectar el tubo flexible de la salida de presión (4) para evitar un consumo no deseado.
Autonomía **
Dependiendo de la lectura de presión en el manómetro y el caudal seleccionado
Bala de 2 litros
Presión en bar | Flujos en L/min | ||||
3 | 6 | 9 | 12 | 15 | |
300 | 3h20min | 1h40min | 66min | 50min | 40min |
250 | 2h46min | 83min | 55min | 41min | 33min |
200 | 2h13min | 66min | 44min | 33min | 26min |
150 | 1h40min | 50min | 33min | 25min | 20min |
50 | 33min | 16min | 11min | 8min | 6min |
**Valor indicativo
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
AIR LIQUIDE SantéINTERNATIONAL – 75, Quai D`Orsay
75007 París (FRANCIA)
Responsables de la fabricación
Air Liquide Medical
Tolhuisstraat 46
2627 Schelle
Bélgica
Fecha de la última revisión de este prospecto: Mayo 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Instrucciones de uso y manipulación
No fumar.
No acercar a una llama.
No engrasar.
En particular:
- No introducir nunca este gas en un aparato que se sospeche pueda contener materias combustibles, en especial si son de naturaleza grasa.
- No limpiar nunca con productos combustibles, en especial si son de naturaleza grasa, ni los aparatos que contienen este gas ni las válvulas, juntas, guarniciones, dispositivos de cierre.
- No aplicar ninguna materia grasa (vaselina, pomadas, etc.) en el rostro de los pacientes.
- No utilizar aerosoles (laca, desodorante, etc.) ni disolventes (alcohol, perfume, etc.) sobre el material o cerca de él.
No exponga nunca la bala a una temperatura superior a 50º C
No fuerce ninguna parte de la bala, en caso de tener problemas póngase inmediatamente en contacto con el fabricante
No sitúe la bala al sol detrás de un cristal
Sujete las balas con cinturones de seguridad para evitar caídas y golpes
Devolver con la presión residual
Cerrar la válvula después del uso
Abrir la válvula lentamente
Mantener siempre el sombrerete de protección
Puede provocar o agravar un incendio
Comburente
Contiene gas a presión: peligro de explosión en caso de calentamiento
Mantener las válvulas y accesorios libres de grasa y aceite
Almacenar alejado de materiales combustibles
En caso de incendio detener la fuga, si no hay peligro de hacerlo
Almacenar en un lugar bien ventilado
Las balas de gas de Oxígeno Medicinal Gas Air Liquide99,5% v/v300 bar están reservadas exclusivamente al uso terapéutico.
Para evitar cualquier incidente, es necesario respetar obligatoriamente las siguientes normas:
- Verificar el buen estado del material antes de su utilización.
- No forzar nunca una bala de gas en un soporte demasiado estrecho para ella.
- Manipular el material con las manos limpias y libres de grasa.
- En el momento de la entrega por parte del fabricante, verificar que la bala de gas está provista de un sistema de garantía de inviolabilidad (precinto) intacto.
- No levantar la bala de gas cogiéndola por la válvula.
- Utilizar conexiones o elementos flexibles de conexión específicos para el oxígeno.
- Utilizar un manorreductor con un caudalímetro que admita una presión de, al menos, 1,5 veces la presión máxima de servicio (300 bares) de la bala de gas (salvo si hay un reductor incorporado a la válvula).
- Utilizar elementos flexibles de conexión en las tomas murales provistos de boquillas específicas para oxígeno.
- Abrir la válvula de forma progresiva.
- No forzar nunca la válvula para abrirla, ni abrirla del todo.
- Purgar la conexión de salida de las balas de gas antes de incorporar el manorreductor para eliminar el polvo que pudiese haber. Mantener limpias las conexiones entre la bala de gas y el manorreductor.
- No someter nunca el manorreductor a varias presurizaciones sucesivas.
- No colocarse nunca frente a la salida de la válvula, sino siempre en el lado opuesto al manorreductor, detrás de la bala de gas y hacia atrás. No exponer nunca a los pacientes al flujo gaseoso.
- No utilizar conexiones intermedias para permitir la conexión de dos dispositivos que no encajan entre sí.
- No intentar reparar una válvula defectuosa.
- No apretar nunca con tenazas el manorreductor-caudalímetro, bajo riesgo de provocar desperfectos en la junta.
- Verificar previamente la compatibilidad de los materiales en contacto con el oxígeno, utilizando en particular juntas de conexión del manorreductor especiales para el oxígeno.
- Cerrar la válvula de la bala de gas tras su utilización, permitir que disminuya la presión del manorreductor dejando abierto el caudalímetro, cerrar el caudalímetro y aflojar a continuación (salvo en el caso de los manorreductores integrados) el tornillo de regulación del manorreductor.
- En caso de fuga, cerrar la válvula de alimentación del circuito que tenga un defecto de estanqueidad, y comprobar que se activa el dispositivo de emergencia.
- No vaciar nunca por completo una bala de gas.
- Conservar las balas de gas vacías con la válvula cerrada (para evitar procesos de corrosión en presencia de humedad en su interior).
- No trasvasar gas bajo presión de una bala de gas a otra.
- Ventilar, si es posible, el lugar de utilización, si se trata de ubicaciones reducidas (vehículos, domicilio).
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OXIGENO MEDICINAL GAS AIR LIQUIDE 99,5% V/V 300 BAR GAS COMPRIMIDO MEDICINALForma farmacéutica: INHALACIÓN PULMONAR, 21-22,5% v/vPrincipio activo: OxigenoFabricante: Nippon Gases Espana SluRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, Oxigeno 21,0% v/v al 22,5% v/vPrincipio activo: OxigenoFabricante: Oxigen Salud S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 100 % oxigenoPrincipio activo: OxigenoFabricante: Linde Gas Espana S.A.U.Requiere receta
Médicos online para OXIGENO MEDICINAL GAS AIR LIQUIDE 99,5% V/V 300 BAR GAS COMPRIMIDO MEDICINAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OXIGENO MEDICINAL GAS AIR LIQUIDE 99,5% V/V 300 BAR GAS COMPRIMIDO MEDICINAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






