
ONDANSETRON BLUEFISH 8 MG COMPRIMIDOS BUCODISPERSABLES EFG

Cómo usar ONDANSETRON BLUEFISH 8 MG COMPRIMIDOS BUCODISPERSABLES EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Ondansetrón Bluefish comprimidos bucodispersables y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Ondansetrón Bluefish comprimidos bucodispersables
- Cómo tomar Ondansetrón Bluefish comprimidos bucodispersables
- Tratamiento y prevención de náuseas y vómitos relacionado con quimioterapia y/o radioterapia
- Prevención de las náuseas y vómitos postoperatorios
- Los comprimidos deben ser tomados como se indica:
- Posibles efectos adversos
- Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Raras (pueden afectar hasta 1 de cada 1.000 personas)
- Muy raras (pueden afectar hasta 1 de cada 10.000 personas)
- Conservación de Ondansetrón Bluefish comprimidos bucodispersables
- Contenido del envase e información adicional
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Ondansetrón Bluefish8 mg comprimidos bucodispersables EFG
ondansetrón
Lea todo el prospecto detenidamente antes de empezar a tomar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Ondansetrón Bluefish comprimidos bucodispersables y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Ondansetrón Bluefish comprimidos bucodispersables
- Cómo tomar Ondansetrón Bluefish comprimidos bucodispersables
- Posibles efectos adversos
- Conservación de Ondansetrón Bluefish comprimidos bucodispersables
- Contenido del envase e información adicional
1. Qué es Ondansetrón Bluefish comprimidos bucodispersables y para qué se utiliza
Ondansetrón Bluefish comprimidos bucodispersables es un comprimido de disolución rápida cuando es colocado en la lengua.
Este medicamento contiene ondansetrón, que pertenece a un grupo de medicamentos llamados antieméticos que se puede emplear para prevenir las náuseas y vómitos.
Ondansetrón comprimidos bucodispersables pueden ser empleados para:
- Parar los efectos de náuseas y vómitos causados por quimioterapia citotóxica en niños y adultos
- Prevenir náuseas y vómitos postoperatorios en niños y adultos
- Parar los efectos de náuseas y vómitos causados por radioterapia en adultos
Si no está seguro porque le han prescrito este tratamiento, consulte a su médico.
2. Qué necesita saber antes de empezar a tomar Ondansetrón Bluefish comprimidos bucodispersables
No tome Ondansetrón Bluefish comprimidis bucodispersables
- si es alérgico a ondansetrón o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si está tomando apomorfina (utilizada para el tratamiento del Parkinson)
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Ondansetrón Bluefish comprimidos bucodispersables:
- si está embarazada o puede quedarse embarazada próximamente,
- si está dando el pecho
- si tiene problemas de hígado
- si tiene obstrucción en el intestino o padece estreñimiento grave
- si se trata de niños menores de 2 años o con una superficie corporal de menos de 0,6 m2.
Otros medicamentos yOndansetrón Bluefish comprimidos bucodispersables
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento como fenitoína, carbamazepina, rifampicina, tramadol.
Embarazo y lactancia
Ondansetrón Bluefish comprimidos bucodispersables no debe utilizarse durante el primer trimestre del embarazo. Esto se debe a que Ondansetrón Bluefish comprimidos bucodispersables puede aumentar ligeramente el riesgo de que un bebé nazca con labio leporino y/o fisura palatina (aberturas o hendiduras en el labio superior o en el paladar). Si ya está embarazada, cree que está podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar Ondansetrón Bluefish comprimidos bucodispersables. Si es una mujer en edad fértil, se le recomienda utilizar un método anticonceptivo efectivo.
Probablemente el ondansetrón puede pasar a la leche. Se recomienda, por tanto, que las madres en periodo de lactancia no amamanten a los niños, si están tomando Ondansetrón Bluefish comprimidos bucodispersables.
Conducción y uso de máquinas
Ondansetrón Bluefish comprimidos bucodispersables no afecta la capacidad de conducir o emplear maquinaria.
Ondansetrón Bluefish comprimidos bucodispersables contiene aspartamo, glucosa, maltodextrina, sorbitol, dióxido de azufre y sodio
Ondansetrón Bluefish comprimidos bucodispersables contiene aspartamo (E 951). Este medicamento contiene 1,76 mg de aspartamo en cada comprimido bucodispersable de 8 mg.
El aspartamo contiene una fuente de fenilalanina que puede ser perjudicial en caso de padecer fenilcetonuria (FCN), una enfermedad genética rara en la que la fenilalanina se acumula debido a que el organismo no es capaz de eliminarla correctamente.
Ondansetrón Bluefish comprimidos bucodispersables contiene sorbitol (E 420). Este medicamento contiene 16,9 mg de sorbitol en cada comprimido bucodispersable de 8 mg.
Ondansetrón Bluefish comprimidos bucodispersables contiene glucosa y maltodextrina. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Puede producir caries.
Ondansetrón Bluefish comprimidos bucodispersables contiene dióxido de azufre (E 220). Este medicamento puede producir reacciones alérgicas graves y broncoespasmo (sensación repentina de ahogo) porque contiene “dióxido de azufre”.
Ondansetrón Bluefish comprimidos bucodispersables contiene sodio. Este medicamento contiene menos de 23 mg de sodio (1mmol) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Ondansetrón Bluefish comprimidos bucodispersables
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Después de iniciado el tratamiento
Los comprimidos bucodispersables de ondansetrón comienzan a actuar a las 1-2 horas. En caso de vomitar antes de transcurrir 1 hora, tome otra dosis idéntica. En caso contrario, no tome más comprimidos ni tampoco reduzca el tiempo entre tomas. Consulte a su médico si persisten las molestias.
Tratamiento y prevención de náuseas y vómitos relacionado con quimioterapia y/o radioterapia
Adultos
La dosis recomendada es de 8mg, 1 a 2 horas antes de la quimioterapia seguido por 8mg cada 12 horas más tarde durante 5 días. El médico decidirá si debe darle una inyección en vez de comprimidos.
Ancianos
La misma dosis que para adultos.
Niños mayores de dos años y adolescentes menores de 18 años:
La dosis es individual y depende del tamaño / superficie corporal del niño. Ondansetrón Bluefish no debe usarse en niños con una superficie corporal total inferior a 0,6 m2.
Niños a partir de 6 meses y adolescentes
- La dosis recomendada es de hasta 4mg dos veces al día
- Puede administrase durante 5 días
Prevención de las náuseas y vómitos postoperatorios
Adultos
La dosis habitual es de 16 mg antes de la anestesia o, alternativamente, 8 mg una hora antes de la anestesia seguida por dosis adicionales de 8 mg a las 8 y 16 horas. El médico decidirá si debe darle una inyección en vez de comprimidos.
Ancianos
Hay experiencia limitada en el uso de ondansetrón en ancianos. Ondansetrón es bien tolerado por pacientes mayores de 65 años con quimioterapia (por favor, véase la sección anterior).
Pacientes con insuficiencia hepática moderada o severa:la dosis diaria total no debe ser de más de 8 mg.
Pacientes con un pobre metabolismo de esparteína/debrisoquina:No precisan alteración ni de la dosis ni de la posología.
No saque los comprimidos ni perfore el blíster hasta que esté listo para tomar el medicamento.
Los comprimidos deben ser tomados como se indica:
No saque Ondansetrón Bluefish comprimidos bucodispersables ni perfore el blíster hasta que esté listo para tomar el medicamento.
Para prevenir la rotura de los comprimidos, es importante no presionar el comprimido hasta que perfore el blíster (Figura A).
Los comprimidos en cada blíster están separados por perforaciones. Separe cada comprimido siguiendo las perforaciones (Figura 1). El film de recubrimiento debe ser eliminado con precaución. Empezando por la esquina marcada con la flecha (Figura 2 y 3).
Los comprimidos deben ser sacados con las manos secas y colocadas sobre la lengua (Figura 4). El comprimido se desintegrará, después de lo cual puede ser ingerido con agua.
Figura A. Figura 1.
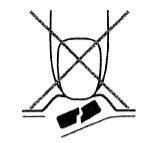
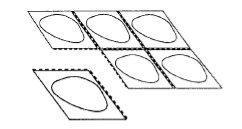
Figura 2. Figura 3.
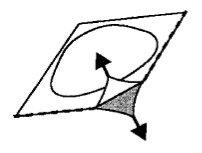
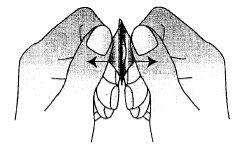
Figura 4.
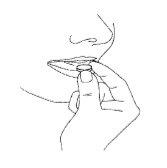
Si toma más Ondansetrón Bluefish comprimidos bucodispersables del que debe
Si usted o su hijo toma más Ondansetrón Bluefish del que debe, hable con un médico o vaya inmediatamente al hospital más cercano. Lleve el medicamento consigo. También puede llamar al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Ondansetrón Bluefish comprimidos bucodispersables
No tome una dosis doble para compensar las dosis olvidadas.
Si olvidó una dosis y siente nauseas o vómitos tome un comprimido de Ondansetrón Bluefish lo antes posible y luego continúe con el tratamiento conforme se le ha indicado. Si olvidó una dosis, pero no siente nauseas o vómitos tome la próxima dosis conforme le haya indicado.
4. Posibles efectos adversos
Al que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunas personas pueden ser alérgicas a los medicamentos, en caso de que apareciera alguno de los síntomas siguientes poco después de tomar Ondansetrón, deje de tomar el medicamento y avise a su médico inmediatamente.
- Aparición súbita de “pitos” y dolor u opresión en el pecho.
- Hinchazón de párpados, cara, labios, boca o lengua.
- Erupción en la piel o urticaria en cualquier parte del cuerpo.
- Colapso
Otros posibles efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Dolorde cabeza.
Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Estreñimiento.
- Sensación de calor o rubor.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Arritmias, Dolor en el pecho con sin depresión del segmento ST, palpitaciones (latido irregular del corazón) o latido lento del corazón.
- Aumento asintomático de los resultados de las pruebas sanguíneas de comprobación de funcionamiento del hígado.
- Convulsiones, movimientos involuntarios del cuerpo incluyendo reacciones extrapiramidales como reacciones distónicas, incluyendo movimientos giratorios ascendentes de los ojos y disquinesia han sido observados sin consecuencias clínicas de relevancia.
- Hipo.
- Presión baja de la sangre (hipotensión).
Raras (pueden afectar hasta 1 de cada 1.000 personas)
- Reacción alérgica inmediata, que puede ser grave, tales como hinchamiento de la boca y garganta que causan dificultad para respirar.
- Se han producido también mareos cuando se administra ondansetrón directamente en vena, que en la mayoría de los casos es prevenido o resuelto con un aumento del periodo de infusión.
- Problemas de la vista (p ej. visión borrosa) asociado con ondansetrón por vía inyectable.
Muy raras (pueden afectar hasta 1 de cada 10.000 personas)
- Pérdida temporal de la vista casi siempre por vía inyectable.
La mayoría de los casos de ceguera se han resuelto a los 20 minutos. La mayoría de los pacientes habían recibido agentes quimioterapéuticos, incluyendo cisplatina. Algunos de los casos de ceguera transitoria notificados eran de origen cortical.
El sabor de fresa contiene dióxido de azufre (E220) que puede causar raras reacciones de hipersensibilidad y broncoespasmo.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
Isquemia miocárdica
Los signos incluyen:
- dolor repentino en el pecho u
- opresión en el pecho
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es . Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ondansetrón Bluefish comprimidos bucodispersables
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en el embalaje original para protegerlo de la luz.
Conservar por debajo de 30ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el estuche y el blíster después de “CAD.”. La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa indicios visibles de deterioro, como descoloración o comprimidos rotos.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de su farmacia habitual. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ondansetrón Bluefish comprimidos bucodispersables
- El principio activo es ondansetrón. Cada comprimido bucodispersable contiene 8 mg de ondansetrón.
- Los demás excipientes son: aspartamo (E951), crospovidona tipo B, estearato de magnesio (E572), celulosa microcristalina (E460), Pharmaburst TM C1, (que contiene manitol (E421), sorbitol (E420), crospovidona (tipo A) y dióxido de silicio coloidal), sabor de fresa, (que contiene glucosa, maltodextrina, goma arábica (E414) 2.3% y dióxido de azufre (E220)), fumarato de estearilo y sodio.
Aspecto del producto ycontenidodel envase de Ondansetrón Bluefish comprimidos bucodispersables
Comprimido bucodispersable
Comprimido bucodispersable, de color blanco, plano, redondo y biselado.
Ondansetrón Bluefish comprimidos bucodispersables se presenta en envases de: 6, 10, 14, 20, 30, 50, 60, 100 comprimidos bucodispersables en blíster de aluminio/OPA//PVC.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercializacióny responsable de fabricación
Titular de la autorización de comercialización:
Bluefish Pharmaceuticals AB
P.O. Box 49013,
100 28 Estocolmo,
Suecia
Responsable de fabricación:
Bluefish Pharmaceuticals AB,
Gävlegatan 22,
113 30 Estocolmo,
Suecia
Sofarimex Industria Química e Farmacêutica S.A.
Av. das Indústrias- Alto do Colaride, Cacem, 2735-213
Portugal
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Bluefish Pharma S.L.U.,
AP 36007
2832094 Madrid, Sucursal 36
Este prospecto está disponible para ciegos o para aquellos con deficiencias de la visión bajo petición.
Este medicamento está autorizado enlos estados miembros del Espacio Económico Europeocon los siguientes nombres
Estado | Nombre del medicamento |
Alemania | Ondansetron Bluefish 8 mg schmelztabletten |
Dinamarca | Ondansetron Bluefish 8 mg smeltetabletter |
España | Ondansetron Bluefish 8 mg comprimidos bucodispersibles EFG |
Noruega | Ondansetron Bluefish 8 mg smeltetabletter |
Polonia | Ondansetron Bluefish |
Reino Unido (Irlanda del Norte) | Ondansetron 8 mg orodispersible tablets |
Suecia | Ondansetron Bluefish 8 mg munsönderfallande tabletter |
Fcehadelaúltimarevisióndeeste prospecto: Junio 2022
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española del Medicamento y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ONDANSETRON BLUEFISH 8 MG COMPRIMIDOS BUCODISPERSABLES EFGForma farmacéutica: INYECTABLE, 2 MG/MLPrincipio activo: OndansetronFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 4 MGPrincipio activo: OndansetronFabricante: Aristo Pharma Iberia S.L.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 8 MGPrincipio activo: OndansetronFabricante: Aristo Pharma Iberia S.L.Requiere receta
Médicos online para ONDANSETRON BLUEFISH 8 MG COMPRIMIDOS BUCODISPERSABLES EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ONDANSETRON BLUEFISH 8 MG COMPRIMIDOS BUCODISPERSABLES EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















