LOMPER 20 mg/ml ORAL SUSPENSION

How to use LOMPER 20 mg/ml ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Lomper 20 mg/ml Oral Suspension
Mebendazole
Read this package leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What Lomper Oral Suspension is and what it is used for
- What you need to know before taking Lomper Oral Suspension
- How to take Lomper Oral Suspension
- Possible side effects
- Storage of Lomper Oral Suspension
- Package Contents and Additional Information
1. What Lomper Oral Suspension is and what it is used for
Lomper is a medicine that belongs to the group of medicines called antihelmintics.
Lomper is indicated for the treatment of the following intestinal parasitoses, both simple and mixed: Enterobiasis (Oxyuriasis), Ascariasis, Trichuriasis, Hookworm disease, and Necatoriasis.
2. What you need to know before taking Lomper Oral Suspension
Do not take Lomper:
- if you are allergic to mebendazole or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
- a possible relationship has been established between the use of mebendazole and metronidazole (a medicine used to treat bacterial and protozoal infections) simultaneously and the occurrence of severe reactions (Stevens-Johnson Syndrome / Toxic Epidermal Necrolysis (blisters or significant skin peeling or in the mouth, eyes, or genital-anal area, with fever)) that can be life-threatening (see section Possible Side Effects)
Children and Adolescents.
It should not be used in children under 1 year of age, as it is not known whether mebendazole is safe in these children. Cases of seizures have been reported in children (very rarely), including children under 1 year of age.
It is not recommended for use in children under 2 years of age, as mebendazole has not been sufficiently studied in these children. Your doctor will assess whether to administer it to children under two years of age, and it will only be administered if the expected benefit justifies the possible risk.
Taking Lomper with Other Medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription.
The use of this medicine together with metronidazole (see Warnings and Precautions) should be avoided.
Concomitant administration with cimetidine (a medicine used to treat stomach acidity) may inhibit the metabolism of mebendazole at the hepatic level, thereby increasing plasma concentrations of the drug. Your doctor will perform the necessary checks to adjust the dose of the medicine.
Taking Lomper with Food
Lomper can be administered with or without food.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Your doctor will assess the possible risks versus the expected therapeutic benefits of administering Lomper during pregnancy, especially during the first trimester.
Breastfeeding
If you are breastfeeding, consult your doctor first, who will decide whether you can take Lomper.
Driving and Using Machines
The influence of this medicine on the ability to drive and use machines is zero or insignificant.
Lomper 20 mg/ml Oral Suspension contains sucrose, ethanol, methyl parahydroxybenzoate (E-218), and propyl parahydroxybenzoate (E-216)
This medicine contains sucrose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine.
This medicine contains 3.75 mg of ethanol (alcohol) per milliliter. The amount in 5 ml of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect. It may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate (E-218) and propyl parahydroxybenzoate (E-216).
This medicine contains less than 1 mmol of sodium (23 mg) per 5 ml; i.e., it is essentially "sodium-free".
This medicine contains fragrances (tutti-frutti essence) with allergen: citral. The allergen citral may cause allergic reactions. In addition to allergic reactions in sensitized patients, sensitization may occur in non-sensitized patients.
3. How to Take Lomper Oral Suspension
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Your doctor will establish the suitable dose for you and make the necessary adjustments.
If you think the action of Lomper is too strong or too weak, tell your doctor or pharmacist. Your doctor will indicate the duration of treatment with Lomper.
The recommended dose is:
- Oxyuriasis:
One 5 ml spoonful of suspension (a single 100 mg dose). It is recommended to repeat the treatment after 2 and 4 weeks.
- Ascariasis, Trichuriasis, Hookworm disease, Necatoriasis, or Mixed Infections:
Two 5 ml spoonfuls per day, one in the morning and one in the afternoon, for three consecutive days.
Use in Children and Adolescents
Children from 2 years of age and adolescents:
- Oxyuriasis:
A single dose of 5 ml of oral suspension (1 measuring spoon).
It is recommended to repeat the treatment after 2 and 4 weeks.
- Ascariasis, Trichuriasis, Hookworm disease, Necatoriasis, or Mixed Infections:
A dose of 5 ml of oral suspension (1 measuring spoon) twice a day, one in the morning and one in the afternoon, for three consecutive days.
Remember to take your medicine.
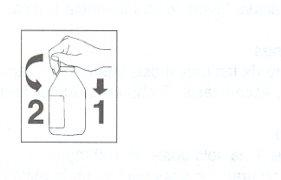
The suspension should be shaken before use. The bottle has a child-resistant cap, and it should be opened as follows: To open the child-resistant cap, press the side down (1) and turn as indicated by the arrow (2). |
|
If you take more Lomper than you should
If you have taken more Lomper than you should, consult your doctor or pharmacist immediately.
In case of accidental overdose, you may experience stomach cramps, nausea, vomiting, and diarrhea.
If you have taken more Lomper than recommended or for extended periods, be aware that you may experience blood disorders, kidney or liver problems, some of which can be serious, as well as hair loss, which can be permanent in some cases.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following side effects occur, stop treatment and consult your doctor:
-Allergic reactions such as blistering skin rash, facial swelling, or difficulty breathing.
-Blisters or significant skin peeling or in the mouth, eyes, or genital-anal area, with fever.
-Seizures
The following side effects have been described. The assessment of side effects is based on the following frequency data:
Very common: may affect more than 1 in 10 people
Common: may affect up to 1 in 10 people
Uncommon: may affect up to 1 in 100 people
Rare: may affect up to 1 in 1,000 people
Very rare: may affect up to 1 in 10,000 people
Frequency not known (cannot be estimated from available data)
Common: abdominal pain.
Uncommon: abdominal discomfort, diarrhea, and flatulence (gas), rash (redness), nausea, and vomiting.
Rare: dizziness.
Very rare: neutropenia (severe decrease in white blood cell count), hypersensitivity reactions (allergic), seizures, liver function changes, hepatitis, toxic epidermal necrolysis, and Stevens-Johnson syndrome (blisters or significant skin peeling or in the mouth, eyes, or genital-anal area, with fever), exanthema (redness), angioedema (facial swelling), urticaria (skin redness with hives), and alopecia (hair loss, which can be permanent in some cases), kidney inflammatory diseases.
Reporting of Side Effects:
If you experience any side effects, talk to your doctor or pharmacist, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Lomper Oral Suspension
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Store in the original packaging.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Lomper Oral Suspension
- The active ingredient of this medicine is mebendazole. The oral suspension contains 20 mg of mebendazole per ml.
- The other ingredients are: sodium saccharin, sucrose, microcrystalline cellulose, and sodium carboxymethylcellulose, sodium lauryl sulfate, methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), quinoline yellow (E-104), methylcellulose, citric acid monohydrate, ethanol (part of the tutti-frutti essence), purified water.
Appearance of the Product and Package Contents
It is presented in a 30 ml plastic bottle with a child-resistant polyethylene cap, with a built-in polyethylene dropper, and a 5 ml measuring spoon made of plastic.
Lomper is a homogeneous yellow suspension.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
Spain
Manufacturer:
TOWA Pharmaceutical Europe, S.L.
C/ de Sant Martí, 75-97
08107 Martorelles (Barcelona)
Spain
Date of the Last Revision of this Package Leaflet: 05/2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price4.4 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LOMPER 20 mg/ml ORAL SUSPENSIONDosage form: TABLET, 100 mgActive substance: mebendazoleManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: TABLET, 400 mgActive substance: albendazoleManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: TABLET, 3 mgActive substance: ivermectinManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for LOMPER 20 mg/ml ORAL SUSPENSION
Discuss questions about LOMPER 20 mg/ml ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions