
GLATIRAMERO VIATRIS 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar GLATIRAMERO VIATRIS 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Glatiramero Viatris 40 mg/ml solución inyectable en jeringa precargada
glatiramero, acetato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Glatiramero Viatris y para qué se utiliza
- Qué necesita saber antes de empezar a usar Glatiramero Viatris
- Cómo usar Glatiramero Viatris
- Posibles efectos adversos
- Conservación de Glatiramero Viatris
- Contenido del envase e información adicional
1. Qué es Glatiramero Viatris y para qué se utiliza
Glatiramero es un medicamento utilizado para el tratamiento de las formas recurrentes de la esclerosis múltiple (EM). Modifica el modo en que funciona el sistema inmunitario de su cuerpo y se clasifica como un agente inmunomodulador. Se cree que los síntomas de la esclerosis múltiple (EM) se producen por un defecto en el sistema inmunitario del organismo. Esto produce zonas de inflamación en el cerebro y en la médula espinal.
Glatiramero se usa para reducir el número de veces que sufre ataques de EM (recaídas). No se ha demostrado que ayude si padece alguna forma de EM que no tiene recaídas o casi ninguna recaída. Glatiramero puede no tener efecto alguno en la duración de un ataque de EM, o en lo mal que lo pasa durante un ataque.
2. Qué necesita saber antes de empezar a usar Glatiramero Viatris
No use Glatiramero Viatris
- Si es alérgico a acetato de glatiramero o a alguno de los demás componentesde este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Glatiramero Viatris:
- Si padece algún problema de riñón o de corazón, ya que podría necesitar hacerse análisis o reconocimientos periódicos.
- Si tiene o ha tenido algún problema de libido (incluidos los debidos al consumo de alcohol).
Glatiramero Viatris puede causar reacciones alérgicas graves, algunas de las cuales pueden ser potencialmente mortales.
Estas reacciones se pueden producir poco después de la administración, incluso meses o años después del inicio del tratamiento e incluso aunque no se hayan producido reacciones alérgicas tras administraciones previas.
Los signos y síntomas de las reacciones alérgicas se pueden superponer con las reacciones tras la inyección. Su médico le informará sobre los signos de una reacción alérgica.
Niños
Glatiramero no se puede utilizar en niños menores de 18 años.
Pacientes de edad avanzada
Glatiramero no se ha estudiado específicamente en personas de edad avanzada. Consulte a su médico al respecto.
Otros medicamentos y Glatiramero Viatris
Informe a su médico o farmacéuticosi está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico para que le oriente sobre el tratamiento con acetato de glatiramero durante el embarazo.
Glatiramero puede utilizarse durante el embarazo siguiendo las recomendaciones de su médico.
Datos limitados en humanos no mostraron efectos negativos del acetato de glatiramero en recién nacidos/lactantes amamantados. El acetato de glatiramero puede utilizarse durante la lactancia.
Conducción y uso de máquinas
Glatiramero no altera la capacidad para conducir o utilizar máquinas.
3. Cómo usar Glatiramero Viatris
Siga exactamente las instrucciones de administración indicadas por su médico para este medicamento. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada en adultos es una jeringa precargada (40 mg de acetato de glatiramero), administrado bajo la piel (por vía subcutánea) tres veces por semana, con una separación entre inyecciones de al menos 48 horas, por ejemplo lunes, miércoles y viernes. Se recomienda administrar el fármaco los mismos días cada semana.
Es muy importante que la inyección de glatiramero se realice correctamente:
- Solo dentro del tejido debajo de la piel (tejido subcutáneo) (ver “Instrucciones de uso” más adelante).
- A la dosis indicada por su médico. Administre únicamente la dosis prescrita por su médico.
- Nunca utilice la misma jeringa más de una vez. Cualquier producto no utilizado o sobrante deberá ser desechado.
- No mezcle o coadministre el contenido de las jeringas precargadas de glatiramero con ningún otro producto.
- Si la solución contiene partículas, no la utilice. Use una jeringa nueva.
La primera vez que utilice glatiramero se le darán instrucciones completas y será supervisado por un médico o una enfermera. Ellos estarán con usted durante la inyección y durante la media hora siguiente, solo para asegurarse de que no tiene ningún problema.
Instrucciones de uso
Lea estas instrucciones con atención antes de usar Glatiramero Viatris.
Antes de la inyección asegúrese de que tiene todo lo que necesita:
- Un blíster con la jeringa precargada de glatiramero.
- Un contenedor para desechar las agujas y jeringas usadas.
- Para cada inyección, saque solo un blíster con una jeringa precargada del envase. Mantenga el resto de las jeringas en la caja.
- Si su jeringa estaba en la nevera, saque el blíster que contiene la jeringa al menos 20 minutos antes de que vaya a inyectarse el medicamento, así se calentará hasta la temperatura ambiente.
Lávese las manos concienzudamente con agua y jabón.
Si desea utilizar un dispositivo para inyección para inyectarse, puede usar el dispositivo para inyección de jeringas precargadas con Glatiramero Viatris . El dispositivo para inyección de jeringas precargadas solamente está aprobado para su uso con Glatiramero Viatris y no se ha probado con otros productos. Consulte las instrucciones de uso proporcionadas con el dispositivo para inyección de jeringas precargadas.
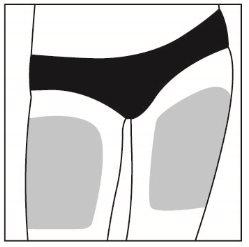
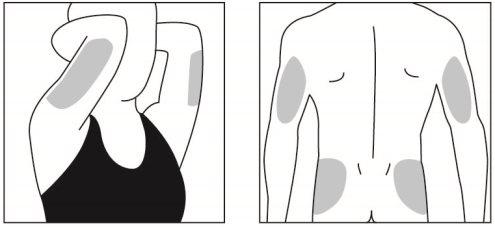
Elija un lugar para la inyección dentro del área siguiendo los diagramas.
Hay siete posibles zonas para la inyección en su cuerpo:
.
Área 1: área del estómago (abdomen) alrededor del ombligo. Evite la zona 5 cm alrededor del ombligo.
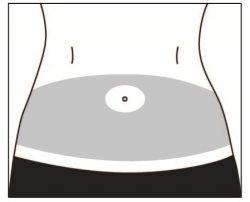
Áreas 2 y 3: Muslos (por encima de las rodillas)
Áreas 4, 5, 6 y 7: Parte posterior de la parte superior de los brazos, final de la parte superior de las caderas (por debajo de la cintura).
Dentro de cada área de inyección hay varios lugares donde se puede aplicar la inyección. Elija un lugar diferente para la inyección cada día. Esto reducirá la posibilidad de irritación o de dolor en el lugar de la inyección. Cambie de lugar para la inyección dentro de cada área. No use siempre el mismo lugar para la inyección.
Advertencia:no se inyecte en ninguna zona que esté dolorida o sin color, o en dónde note nudos o bultos firmes. Se recomienda tener un esquema con los lugares para la inyección planificados y anotarlo en un diario. Existen algunos lugares en su cuerpo que pueden ser difíciles para la auto-inyección (como la parte de atrás de su brazo). Si quiere usarlos, puede necesitar ayuda.
Cómo inyectar:
- Saque la jeringa del blíster protector, despegando la lámina posterior del blíster.
- Quite el capuchón de la aguja, nouse la boca ni los dientes para hacerlo.
- Pellizque suavemente la piel haciendo un pliegue entre el dedo pulgar e índice (Figura 1).
- Introduzca la aguja en la piel, tal y como se muestra en la Figura 2.
- Inyecte el medicamento empujando el émbolo, firmemente hasta el tope quedando la jeringa vacía.
- Saque la jeringa y la aguja.
- Deseche la jeringa en un contenedor seguro para productos desechables. No tire las jeringas usadas a la basura, deposítelas cuidadosamente en un contenedor a prueba de pinchazos como le ha recomendado su médico o enfermera.
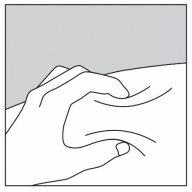
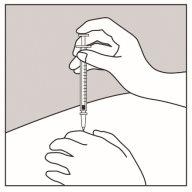
Figura 1 Figura 2
Si tiene la impresión de que el efecto de glatiramero es demasiado fuerte o débil, comuníqueselo a su médico.
Si usa más Glatiramero Viatris del que debe
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Glatiramero Viatris
Adminístrelo en cuanto lo recuerde o tenga la posibilidad de administrarlo, y evite el uso el día siguiente. No se administre una dosis doble para compensar las dosis olvidadas. Si es posible, debería volver a su calendario de administración habitual en la siguiente semana.
Si interrumpe el tratamiento con Glatiramero Viatris
No deje de usar glatiramero sin consultar con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas (hipersensibilidad, reacción anafiláctica)
Usted puede desarrollar una reacción alérgica grave a este medicamento, poco después de la administración. Este es un efecto adverso poco frecuente. Estas reacciones se pueden producir meses o años después del inicio del tratamiento con Glatiramero Viatris, incluso aunque no se hayan producido reacciones alérgicas tras administraciones previas.
Si nota cualquiera de los siguientes efectos adversos repentinos, deje de utilizar glatiramero y llame inmediatamente a su médico o diríjase al servicio de urgencias del hospital más cercano:
- Sarpullido generalizado (manchas rojas o ronchas),
- inflamación de los párpados, la cara,los labios, la boca, la garganta o la lengua
- repentina falta de aliento, dificultad para respirar o sibilancias («pitos» al respirar)
- convulsiones (crisis)
- problemas para tragar o hablar
- síncope (desfallecimiento), sensación de mero o de desmayo
- colapso
Otras reacciones tras la inyección (reacción inmediatamente después de la inyección)
Algunas personas pueden tener uno o más de los siguientes síntomas minutos después de la inyección de acetato de glatiramero. Estos normalmente no suponen ningún problema y desaparecen en media hora.
No obstante, si los siguientes síntomas duran más de 30 minutos, contacte inmediatamente con su médico, o diríjase al servicio de urgencias del hospital más próximo:
- rubor (enrojecimiento) del pecho o la cara (vasodilatación),
- dificultad para respirar (disnea),
- dolor de pecho
- latidos del corazón rápidos y fuertes (palpitaciones, taquicardia).
Problemas hepáticos
Problemas hepáticos o empeoramiento de los problemas hepáticos, incluyendo insuficiencia hepática (que en algunos casos llevó al trasplante hepático), pueden ocurrir raramente con Glatiramero Viatris.
Contacte inmediatamente con su médico si tiene síntomas como:
- náuseas,
- pérdida de apetito,
- orina de color oscuro y heces pálidas,
- coloración amarillenta de la piel o de la parte blanca del ojo,
- sangrado más fácil de lo normal.
En general, los efectos adversos notificados por los pacientes usando acetato de glatiramero 40 mg/ml tres veces a la semana fueron también notificados por los pacientes que usaban acetato de glatiramero 20 mg/ml (remítase a la siguiente lista).
Se han descrito los siguientes efectos adversos con acetato de glatiramero:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- infecciones, gripe
- ansiedad, depresión
- dolor de cabeza
- náuseas
- erupción en la piel
- dolor en las articulaciones o la espalda
- sensación de debilidad, reacciones de la piel en el lugar de la inyección que incluyen, enrojecimiento de la piel, dolor, formación de ampollas, picor, hinchazón de los tejidos, inflamación e hipersensibilidad (estas reacciones en el lugar de la inyección no son anormales y normalmente desaparecen con el tiempo), dolor inespecífico.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- inflamación del tracto respiratorio, gripe de estómago, calenturas, inflamación de los oídos, moqueo nasal, abscesos dentales, candidiasis vaginal
- crecimientos en la piel no malignos (neoplasias benignas de la piel), crecimiento de tejido (neoplasia)
- hinchazón de los ganglios linfáticos
- reacciones alérgicas
- pérdida del apetito, ganancia de peso
- nerviosismo
- alteración del sentido del gusto, aumento de la opresión del tono muscular, migraña, problemas en el habla, desmayo, temblor
- visión doble, problemas en los ojos
- problemas de oído
- tos, fiebre del heno
- problemas anales o del recto, estreñimiento, dientes con caries, indigestión, dificultad para tragar, incontinencia intestinal, vómitos.
- resultados anormales de las pruebas de la función hepática
- cardenales, sudoración excesiva, picor, alteraciones en la piel, urticaria
- dolor en el cuello
- necesidad de vaciar rápidamente su vejiga, orinar frecuentemente, incapacidad para vaciar adecuadamente su vejiga
- resfriado, hinchazón de la cara, pérdida de tejido bajo la piel en el lugar de la inyección, reacción local, hinchazón periférica por acumulación de líquidos, fiebre.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- abscesos, inflamación de la piel y del tejido blando inferior, forúnculos, herpes, inflamación de los riñones
- cáncer de piel
- aumento de la cantidad de glóbulos blancos, disminución de la cantidad de glóbulos blancos, agrandamiento del bazo
- disminución de la cantidad de plaquetas, cambio en la forma de los glóbulos blancos
- agrandamiento del tiroides o hiperactividad del tiroides
- baja tolerancia al alcohol, gota, aumento de los niveles de grasas en la sangre, aumento del sodio en sangre, disminución de la ferritina en sangre.
- sueños extraños, confusión, estado eufórico, ver, oír, oler, tocar o sentir cosas que no están ahí (alucinaciones), agresividad, estado anormalmente feliz, trastornos de personalidad, intento de suicidio
- entumecimiento de las manos y dolor (síndrome del túnel carpiano), trastornos mentales, crisis (convulsión), problemas para escribir y leer, trastornos musculares, problemas con el movimiento, espasmos musculares, inflamación de los nervios, conexión anormal nervio-muscular que produce una función muscular anormal, movimiento rápido e involuntario de los globos oculares, parálisis, pie caído (parálisis del nervio peroneo), estado de inconsciencia (estupor), manchas visuales ciegas
- cataratas, lesiones oculares en la córnea, sequedad ocular, sangrado en el ojo, párpado superior caído, dilatación de la pupila, desgaste del nervio óptico que produce problemas visuales.
- latidos cardiacos de más, latidos cardiacos lentos, episodios de latidos cardiacos rápidos
- varices
- paradas periódicas de la respiración, sangrado de la nariz, respiración anormalmente rápida o profunda (hiperventilación), sensación de estrechamiento de la garganta, problemas en los pulmones, incapacidad para respirar por estrechamiento de la garganta (sensación de asfixia)
- inflamación del intestino delgado, pólipos en el colon, inflamación del intestino, eructos, úlcera en el esófago, inflamación de las encías, sangrado rectal, agrandamiento de las glándulas salivares
- cálculos biliares, agrandamiento del hígado
- hinchazón de la piel y tejidos blandos, sarpullido en la piel por contacto, bultos en la piel enrojecidos dolorosos, bultos en la piel
- hinchazón, inflamación y dolor de las articulaciones (artritis u osteoartritis), inflamación y dolor de las bolsas de líquido que cubren las articulaciones (existentes en algunas de las articulaciones), dolor en el costado, disminución de la masa muscular
- sangre en la orina, piedras en los riñones, problemas en el sistema urinario, anomalías en la orina
- hinchazón de los pechos, dificultad para la erección, caída o desplazamiento de los órganos pélvicos (prolapso pélvico), erecciones mantenidas, alteraciones de la próstata, prueba de Papanicoláu con resultados anormales (frotis anormal de cérvix), alteraciones en los testículos, sangrado vaginal, trastorno vaginal
- quiste, resaca, temperatura corporal más baja de lo normal (hipotermia), inflamación no específica, destrucción de tejidos en el lugar de la inyección, problemas en las membranas mucosas
- alteraciones tras la vacunación.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Glatiramero Viatris
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en nevera (entre 2°C y 8°C).
Las jeringas precargadas de Glatiramero Viatris pueden conservarse hasta un mes fuera de nevera, entre 15ºC y 25ºC. Solo puede hacer esto una vez. Si después de un mes, las jeringas precargadas de Glatiramero Viatris no han sido utilizadas y se encuentran todavía en su envase original, deben volver a conservarse en nevera.
No congelar.
Conservar las jeringas precargadas en el embalaje exterior para protegerlas de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el estuche de cartón después de “CAD”. Los dos primeros dígitos indican el mes y los cuatro últimos dígitos indican el año. La fecha de caducidad es el último día del mes que se indica.
Deseche cualquier jeringa que contenga partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deGlatiramero Viatris
- El principio activo es acetato de glatiramero. 1 ml de la solución para inyección (el contenido de 1 jeringa precargada) contiene 40 mg de acetato de glatiramero.
- Los otros componentes son manitol (E421) y agua para inyectables.
Aspecto del producto y contenido del envase
Glatiramero Viatris es una solución estéril, transparente, incolora o ligeramente amarillenta/amarronada.
Si la solución contiene partículas, deséchela y empiece de nuevo. Use una jeringa nueva.
3 jeringas precargadas
12 jeringas precargadas
36 (3x12) jeringas precargadas
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Viatris Limited
Damastown Industrial Park
Mulhuddart, Dublín 15
Dublín
Irlanda
Responsable de la fabricación
Synthon Hispania S.L.
Polígono Les Salines, C/ Castelló 1
08830 Sant Boi de Llobregat (Barcelona)
España
O
Synthon BV
Microweg 22
6545 CM Nijmegen
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Viatris Pharmaceuticals, S.L.U.
C/ General Aranaz, 86
28027 - Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeoy en el Reino Unido (Irlanda del Norte)con los siguientes nombres:
Alemania: CLIFT 40 mg/ml Injektionslösung in einer Fertigspritze
Bélgica: Glatiramyl 40 mg/ml, oplossing voor injectie in een voorgevulde spuit
Chipre: Glatiramer/Mylan 40 mg/mL εν?σιμο δι?λυμα σε προγεμισμ?νη σ?ριγγα
Dinamarca: Copemyl
España: Glatiramero Viatris 40 mg/ml solución inyectable en jeringa precargada
Finlandia: Glatimyl 40 mg/ml injektioneste, liuos, esitäytetty ruisku
Francia: GLATIRAMER VIATRIS 40 mg/ml, solution injectable en seringue préremplie
Grecia: Glatiramer / Mylan 40 mg/ml solution for injection, pre-filled syringe
Irlandia: Brabio 40 mg/ml solution for injection, pre-filled syringe
Italia: COPEMYLPLUS
Noruega: Copemyl 40 mg/ml injeksjonsvæske, oppløsning i ferdigfylt sprøyte
Países bajos: Glatirameeracetaat Viatris 40 mg/ml, oplossing voor injectie in een voorgevulde spuit
Portugal: Acetato de glatirâmero Mylan
Reino Unido (Irlanda del Norte): Brabio 40 mg/ml solution for injection, pre-filled syringe
Suecia: Glatimyl 40 mg/ml injektionsvätska, lösning, förfylld spruta
El autoinyector reutilizable está autorizado en los estados miembros Espacio Económico Europeo con los siguientes nombres:
Myject
Fecha de la última revisión de este prospecto:noviembre 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GLATIRAMERO VIATRIS 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 20 mg acetato glatiramero/ mlPrincipio activo: Glatiramero acetatoFabricante: Teva GmbhRequiere recetaForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: Glatiramero acetatoFabricante: Teva GmbhRequiere recetaForma farmacéutica: INYECTABLE, 20 mg/mlPrincipio activo: Glatiramero acetatoFabricante: Viatris LimitedRequiere receta
Médicos online para GLATIRAMERO VIATRIS 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GLATIRAMERO VIATRIS 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















