FYDRANE 0,2 MG/ML + 3,1 MG/ML + 10 MG/ML SOLUCION INYECTABLE


Cómo usar FYDRANE 0,2 MG/ML + 3,1 MG/ML + 10 MG/ML SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
FYDRANE0,2 mg/ml +3,1 mg/ml +10 mg/ml, solución inyectable
tropicamida /fenilefrina hidrocloruro / lidocaína hidrocloruro monohidrato
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es FYDRANE y para qué se utiliza
- Qué necesita saber antes de empezar a usar FYDRANE
- Cómo usar FYDRANE
- Posibles efectos adversos
- Conservación de FYDRANE
- Contenido del envase e información adicional
1. Qué es FYDRANE y para qué se utiliza
Qué es FYDRANE
Este medicamento es una solución para inyectar en el ojo.
Contiene tres principios activos:
- Tropicamida que pertenece al grupo de medicamentos que bloquean el paso de los impulsos a través de los nervios (conocidos como anticolinérgicos),
- Fenilefrina (como fenilefrina hidrocloruro) que pertenece a un grupo de medicamentos que mimetizan los efectos de los impulsos transmitidos a través de determinados nervios (que pertenecen a los alfa-simpaticomiméticos),
- Lidocaína (como lidocaína hidrocloruro monohidrato) que pertenece a un grupo de medicamentos llamados anestésicos locales de tipo amida.
Para qué se utiliza
Este medicamento se utiliza únicamente en adultos.
Será administrado por su cirujano oftalmólogo mediante una inyección en el ojo al inicio de la cirugía de cataratas (opacidad del cristalino) para dilatar la pupila de su ojo (midriasis) y obtener anestesia en su ojo durante el procedimiento quirúrgico.
2. Qué necesita saber antes de empezar a usar FYDRANE
No le debe ser administrado FYDRANE:
- si es alérgico a la tropicamida, fenilefrina hidrocloruro y/o lidocaína hidrocloruro monohidrato o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6),
- si es alérgico a anestésicos de tipo amida (como articaina, bupivacaina, mepivacaina, prilocaina, ropivacaina),
- si es alérgico a derivados de la atropina.
Advertencias y precauciones
FYDRANE no está recomendado:
- en cirugía de cataratas combinada con cierto tipo de cirugía del ojo (vitrectomía),
- si la parte anterior (cámara anterior) de su ojo es poco profunda,
- si ha sufrido alguna vez un aumento agudo de la presión ocular (glaucoma agudo de ángulo cerrado).
Consulte con su médico si tiene:
- presión arterial elevada (hipertensión)
- engrosamiento de las paredes arteriales (aterosclerosis)
- cualquier enfermedad cardiaca y, particularmente, si ésta afecta a la frecuencia cardiaca,
- una contraindicación a medicamentos que aumentan la presión arterial (aminas vasopresoras: epinefrina, norepinefrina, dopamina, dobutamina) por vía sistémica,
- glándula tiroides hiperactiva (hipertiroidismo),
- trastornos de la glándula prostática,
- convulsiones (epilepsia),
- cualquier enfermedad del hígado o problemas de riñón,
- cualquier problema respiratorio,
- pérdida de función muscular y debilidad (miastenia gravis).
Uso de FYDRANE con otros medicamentos
Comunique a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Este medicamento no debe ser utilizado:
- durante el embarazo
- durante la lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
FYDRANE tiene una influencia moderada sobre la capacidad de conducir y utilizar máquinas. Por consiguiente, no debe conducir y/o utilizar máquinas hasta que su visión sea normal.
FYDRANE contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar FYDRANE
Sólo debe ser administrado este medicamento si ya ha demostrado, en una evaluación preoperatoria, una dilatación pupilar satisfactoria con un midriático tópico.
Dosis y método de administración
- FYDRANE solución para inyección debe ser administrado por un cirujano oftalmólogo, con anestesia local, al inicio de la cirugía de cataratas.
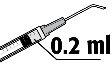
- La dosis recomendada es de 0,2 ml de solución en una única inyección. No deben inyectarse dosis adicionales ya que no se han demostrado efectos acumulativos y debido a que se ha observado un aumento en la pérdida de células endoteliales (células de la capa que recubren la superficie posterior de la córnea).
- La misma dosis es utilizada en adultos y en personas de edad avanzada.
Si le administran demasiada cantidad, o demasiada poca, FYDRANE:
Su medicación será administrada por un cirujano oftalmólogo. Es improbable que le administren una sobredosis. Una sobredosis puede causar pérdida de células endoteliales de la córnea (células de una capa que cubre la superficie posterior de la córnea).
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede tener efectos adversos, aunque no todas las personas los sufran.
Las complicaciones conocidas más graves ocurren durante o después de la cirugía de cataratas:
Poco frecuentes: pueden afectar a hasta 1 de cada 100 personas
- Lesión en la lente (rotura de la cápsula posterior),
- Inflamación de la retina (edema macular cistoide).
Por favor, solicite atención médica urgente en estos casos.
Otros efectos adversos:
Poco frecuentes: pueden afectar a hasta 1 de cada 100 personas
- Dolor de cabeza,
- Inflamación de la córnea (queratitis), aumento de la presión en el ojo, enrojecimiento del ojo (hiperemia ocular),
- Presión arterial elevada (hipertensión).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www. notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de FYDRANE
Mantener fuera de la vista y del alcance de los niños.
No use este medicamento después de la fecha de caducidad que aparece en la caja, blíster y ampolla después de “CAD.”. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Para el uso en un único ojo. Este medicamento debe ser utilizado inmediatamente después de abrir la ampolla.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deFYDRANE
- Los principios activos son tropicamida 0,04 mg, fenilefrina hidrocloruro 0,62 mg y lidocaína hidrocloruro monohidrato 2 mg por cada dosis de 0,2 ml, equivalente a 0,2 mg de tropicamida, 3,1 mg de fenilefrina hidrocloruro y 10 mg de lidocaína hidrocloruro monohidrato para 1 ml.
- Los demás componentes son: cloruro de sodio, fosfato disódico dodecahidrato, fosfato disódico dihidrato, edetato de disodio y agua para preparaciones inyectables.
Aspecto del producto y tamaño del envase
FYDRANE es una solución para inyección transparente, de color amarillo a ligeramente pardusco y prácticamente libre de partículas visibles, envasado en una ampolla de vidrio topacio de 1 ml. Cada ampolla estéril contiene 0,6 ml de la solución para inyección y se presenta sola o junto con una aguja con filtro estéril de 5 micras en un blíster sellado de papel/PVC.
Cada envase contiene 1, 20 ó 100 ampollas estériles (con una aguja con filtro estéril de 5 micrómetros). La aguja con filtro de 5 micras debe ser utilizada únicamente para extraer el contenido del vial.
Todos los componentes son para un único uso.
No todos los tamaños de envase pueden estar comercializados.
Titular de la autorización de comercialización
LABORATOIRES THEA
RUE LOUIS BLÉRIOT, 12
F-63017 CLERMONT-FERRAND CEDEX 2
FRANCIA
Responsable de la fabricación
DELPHARM TOURS
RUE PAUL LANGEVIN
37170 CHAMBRAY LES TOURS
FRANCIA
O
LABORATOIRES THEA
RUE LOUIS BLÉRIOT, 12
F-63017 CLERMONT-FERRAND CEDEX 2
FRANCIA
Representante local:
LABORATORIOS THEA, S.A.
C/ Enric Granados, nº 86-88, 2ª planta
08008 Barcelona
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Bélgica, Bulgaria, Chipre, Croacia, República Checa, Dinamarca, Finlandia, Francia, Alemania, Grecia, Islandia, Italia, Luxemburgo, Holanda, Polonia, Portugal, Rumanía, República Eslovaca, Eslovenia, Suecia, Reino Unido………………………………………….……….Mydrane
Irlanda,España………………………………………………………………………………….Fydrane
Noruega……………………………………………………………........................................Mydane
Fecha de la última revisión de este prospecto: Septiembre 2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS): http://www.aemps.es/
Esta información está destinada únicamente a profesionales del sector sanitario:
Incompatibilidades
En la literatura no se han notificado incompatibilidades de los principios activos con la mayoría de productos utilizados en la cirugía de cataratas, y tampoco durante los ensayos clínicos. Para los viscoelásticos habituales, esto ha sido también confirmado por pruebas de interacción farmacológica.
Advertencias
No utilizar si el blíster está dañado o roto. Abrir únicamente en condiciones de asepsia. La esterilidad del contenido del blíster está garantizada.
Cómo preparar y administrar FYDRANE
Uso único de la solución para un ojo por vía intracameral únicamente.
FYDRANE debe administrarse mediante inyección intraocular en la cámara anterior del ojo (inyección intracameral), por un cirujano oftalmólogo, en las condiciones asépticas recomendadas para la cirugía de cataratas.
Antes de la inyección intracameral, la solución debe ser visualmente inspeccionada y sólo debe ser utilizada si es una solución transparente con un ligero color amarillo a ligeramente pardusco y prácticamente libre de partículas visibles.
La dosis recomendada es de 0, 2 ml de FYDRANE; no se debe inyectar una dosis adicional ya que no se han demostrado efectos acumulativos significativos y porque se observó un aumento de pérdida de células del endotelio.
El producto debe ser utilizado inmediatamente después de abrir la ampolla y no debe ser reutilizado para el otro ojo o para cualquier otro paciente.
Únicamente para la presentación en kit (es decir, blíster que contiene una ampolla y una aguja): pegar la etiqueta desprendible del blíster en la historia del paciente.
Para preparar FYDRANEpara la administración intracameral, siga por favor las siguientes instrucciones: | |
|
|
Después de su uso, eliminar debidamente la solución restante. No debe conservarse para un uso posterior. |
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local. Desechar las agujas utilizadas en un contenedor para materiales punzantes.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FYDRANE 0,2 MG/ML + 3,1 MG/ML + 10 MG/ML SOLUCION INYECTABLEForma farmacéutica: IMPLANTE OFTALMICO, 5,376mg Chloridrato fenilefrina/0,28mg TropicamidaPrincipio activo: tropicamide, combinationsFabricante: Laboratoires TheaRequiere recetaForma farmacéutica: COLIRIO, -Principio activo: atropineFabricante: Alcon Healthcare S.A.Requiere recetaForma farmacéutica: COLIRIO, 5 mgPrincipio activo: atropineFabricante: Alcon Healthcare S.A.Requiere receta
Médicos online para FYDRANE 0,2 MG/ML + 3,1 MG/ML + 10 MG/ML SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FYDRANE 0,2 MG/ML + 3,1 MG/ML + 10 MG/ML SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes