
COLIROFTA ATROPINA 5 MG/ML COLIRIO EN SOLUCION

Cómo usar COLIROFTA ATROPINA 5 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el usuario
COLIROFTA ATROPINA 5 mg/ml colirio en solución
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
|
Contenido del prospecto
- Qué es COLIROFTA ATROPINA 5 mg/ml y para qué se utiliza
- Qué necesita saber antes de empezar a usar COLIROFTA ATROPINA 5 mg/ml
- Cómo usar COLIROFTA ATROPINA 5 mg/ml
- Posibles efectos adversos
5 Conservación de COLIROFTA ATROPINA 5 mg/ml
- Contenido del envase e información adicional
1. Qué es COLIROFTA ATROPINA 5 mg/ml y para qué se utiliza
Es un colirio que contiene atropina, un agente anticolinérgico (bloquea alguno de los receptores de la acetilcolina, un neurotransmisor) que administrado en los ojos produce midriasis (dilatación de la pupila) y cicloplejía (parálisis del músculo que produce la acomodación).
Colirofta Atropina 5 mg/ml está indicado:
- En el examen de los ojos, para dilatar la pupila y evitar el enfoque ocular.
- Para el tratamiento de afecciones oculares inflamatorias agudas de la parte anterior del ojo, como la inflamación de la parte coloreada del ojo (iritis) y del iris y el cuerpo ciliar (iridociclitis).
2. Qué necesita saber antes de empezar a usar COLIROFTA ATROPINA 5 mg/ml
No use COLIROFTA ATROPINA 5 mg/ml
- Si es alérgico a la atropina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene o cree que puede tener glaucoma primario o predisposición a glaucoma de ángulo estrecho (aumento de la presión en el ojo).
- Niños con síndrome de Down, parálisis espástica (un tipo de parálisis cerebral) o lesión cerebral.
- Lactantes menores de 3 meses de edad.
- Niños que hayan tenido una reacción anterior grave al principio activo de este medicamento, la atropina.
(Ver en la sección 2 el apartado “Embarazo, lactancia y fertilidad”).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Colirofta Atropina 5 mg/ml.
- Utilice este medicamento sólo en su(s) ojo(s).
- Tras la aplicación mantenga los ojos cerrados mientras presiona suavemente con un dedo el canal lagrimal durante al menos 1 minuto. Ver sección 3 “Cómo usar…”.
- El uso de este medicamento puede causar:
- Presión aumentada en el ojo y glaucoma, especialmente en pacientes de edad avanzada. Debe controlarse la presión del ojo con frecuencia y también antes de iniciar el tratamiento, con la supervisión del médico.
- Cambios en el comportamiento, (como delirio), especialmente en niños y pacientes de edad avanzada, aunque estas reacciones pueden ocurrir a cualquiera edad.
- Sensibilidad a la luz. Proteger los ojos frente a la luz intensa (gafas de sol).
- Visión borrosa que puede durar hasta 2 semanas.
- Si tiene fiebre o está expuesto a temperaturas altas, especialmente en niños, se requiere precaución, ya que este medicamento puede aumentar la temperatura corporal.
- Se necesita gran precaución en pacientes con enfermedades del corazón, arritmias o infarto de miocardio reciente, ya que se podrían producir taquicardias (aumento de la frecuencia de los latidos). Se recomienda reducir la dosis.
Se podría producir elevación de la presión arterial.
- Se requiere precaución en pacientes con:
- enfermedad del riñón significativa
- enfermedades pulmonares crónicas
- o con síntomas del tracto urinario inferior, como hiperplasia de próstata.
- No debe utilizar este medicamento a la vez que un tratamiento con medicamentos IMAO (antidepresivos generalmente) (ver apartado “Otros medicamentos …”).
Niños
- Este medicamento se utilizará con precaución en lactantes mayores de 3 meses de edad.
- Niños prematuros y con bajo peso al nacer o pacientes con: síndrome de Down, parálisis espástica, lesión cerebral, o niños de piel clara y ojos azules son especialmente sensibles a los efectos adversos de este medicamento. Consulte a su médico para que le informe sobre las reacciones adversas graves que pueden ocurrir con el uso de este medicamento.
- Evite que los niños se pongan este medicamento en la boca o mejillas. Lávese las manos y las mejillas o manos de los niños inmediatamente después de administrarlo.
Otros medicamentos yCOLIROFTA ATROPINA 5 mg/ml
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Especialmente, informe a su médico si está utilizando:
Medicamentos que comparten propiedades con el principio activo del medicamento, como:
- Amantadina (medicamento antiviral y para la enfermedad de Parkinson, que estimula el sistema nervioso central)
- Para enfermedades pulmonares obstructivas, como: tiotropio, glicopirronio o revefenacina
- Escopolamina (utilizado previamente a la anestesia)
- Algunos antihistamínicos (medicamentos usados para tratar alergia)
- Antipsicóticos (usados para tratar afecciones psiquiátricas)
- Antidepresivos tricíclicos (medicamentos usados para tratar depresión).
Otros medicamentos:
- Donepezilo (utilizado en la enfermedad de Alzheimer)
- Estimulantes del corazón
- Medicamentos IMAO (en general, para tratar la depresión).
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Este medicamento no está recomendado durante el embarazo ni durante la lactancia.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad de conducir y utilizar máquinas es importante
Este medicamento puede producir visión borrosa y sensibilidad a la luz durante un tiempo prolongado que puede durar varios días. No conduzca ni utilice máquinas o herramientas hasta que su visión sea clara.
COLIROFTA ATROPINA 5 mg/ml contieneparahidroxibenzoato de metilo (E-218) y parahidroxibenzoato de propilo (E-216) y fosfatos
Este medicamento puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo (E-218) y parahidroxibenzoato de propilo (E-216).
Este medicamento contiene 3,3 mg de fosfatos en cada ml.
Si sufre daño grave en la córnea (la capa transparente de la parte frontal del ojo) el tratamiento con fosfatos, en casos muy raros, puede provocar visión borrosa por acumulación de calcio.
3. Cómo usar COLIROFTA ATROPINA 5 mg/ml
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es:
Adultos
Cuando se pretenda un efecto sostenido, se realizarán en general tres aplicaciones diarias de 2 gotas.
Para el examen de refracción, se instilarán 1 ó 2 gotas en cada ojo dos veces al día, de 1 a 3 días antes de la exploración.
Usoen niños
Niños (mayores de 3 meses de edad)
Debido al riesgo de producir efectos adversos graves sistémicos, este medicamento está contraindicado en lactantes menores de 3 meses de edad y se recomienda utilizar con precaución en lactantes mayores de 3 años. Se recomienda utilizar la dosis eficaz más baja posible para disminuir el riesgo de aparición de efectos adversos sistémicos (ver apartado “Advertencias y precauciones”).
Cuando se pretenda un efecto sostenido, se realizarán en general tres aplicaciones diarias de 1 gota.
Para el examen de refracción, se instilarán 1 o 2 gotas en cada ojo dos veces al día, de 1 a 3 días antes de la exploración.
Recuerde aplicarse su medicamento de acuerdo a las indicaciones de su médico.
Uso en pacientes de edad avanzada
Utilizar con precaución en los pacientes de edad avanzada, ya que pueden tener más riesgo de glaucoma no diagnosticado y trastornos de la conducta.
Su médico le indicará la duración de su tratamiento con Colirofta Atropina 5 mg/ml. No suspenda el tratamiento antes, a menos que su médico se lo indique.
Recomendaciones de uso:
Vía oftálmica (en los ojos).
1 2 3
- Lávese las manos.
- Coja el frasco.
- Después de abrir el frasco por primera vez, debe retirar el anillo de plástico del precinto si está suelto.
- Sostenga el frasco, boca abajo, entre los dedos.
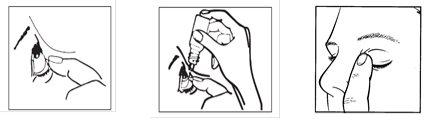
- Incline la cabeza hacia atrás. Separe suavemente el párpado del ojo con un dedo hasta que se forme una bolsa entre el párpado y su ojo, en la que deberá caer la gota (figura 1).
- Acerque la punta del frasco al ojo. Puede serle útil un espejo.
- No toque el ojo o el párpado, zonas próximas ni otras superficies con el cuentagotas. Las gotas podrían contaminarse.
- Apriete suavemente la base del frasco con el dedo índice para que caiga una gota cada vez (figura 2).
- Después de utilizar este colirio, suelte el párpado inferior, cierre su ojo y presione suavemente con el dedo el borde del ojo, junto a la nariz durante al menos 1 minuto. Esto ayuda a evitar que este medicamento pase al resto del cuerpo (figura 3).
- Si se aplica gotas en ambos ojos, repita todos los pasos anteriores con el otro ojo.
- Cierre bien el frasco inmediatamente después de utilizarlo.
- Recuerde lavar sus manos después de la administración de este medicamento.
Si una gota cae fuera del ojo, inténtelo de nuevo.
Si está utilizando otros medicamentos oftálmicos, espere al menos 5 minutos entre la administración de este colirio y los otros medicamentos oftálmicos. Las pomadas oftálmicas deben administrarse en último lugar.
Si usa más COLIROFTA ATROPINA 5 mg/ml del que debe
Puede eliminarlo lavando los ojos con agua templada. No se aplique más gotas hasta que vuelva a tocarle. Los síntomas de sobredosis pueden incluir enrojecimiento y sequedad de la piel (en niños puede presentarse una erupción), visión borrosa, pulso rápido e irregular, fiebre, hinchazón abdominal en niños, convulsiones, alucinaciones, pérdida de coordinación y depresión respiratoria progresiva rápida.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica. Teléfono: 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar COLIROFTA ATROPINA 5 mg/ml
No se aplique una dosis doble para compensar las dosis olvidadas.
Aplíquese una única dosis tan pronto como se dé cuenta y continúe con la siguiente dosis que estaba prevista. Sin embargo, si ya es casi la hora de la siguiente dosis, no se aplique la dosis olvidada y continúe con la siguiente dosis de su régimen habitual.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han notificado durante la experiencia postcomercialización las siguientes reacciones:
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Efectos en el ojo: visión borrosa, incremento en el tamaño de la pupila (efecto prolongado del medicamento), hinchazón del párpado, sensibilidad a la luz.
- Efectos generales: alergia, incremento o disminución de la frecuencia cardíaca, dolor de cabeza, mareo, alucinación, estado de confusión, desorientación, obstrucción intestinal, hinchazón abdominal, vómitos, enrojecimiento o inflamación de la piel, erupción, fiebre.
Otros efectos adversos en niños
Los niños son más propensos a manifestar los efectos adversos generales descritos anteriormente especialmente en niños prematuros y con bajo peso al nacer o pacientes con síndrome de Down, parálisis espástica o lesión cerebral (ver en la sección 2, el apartado “No use…”).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaRAM.es.Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de COLIROFTA ATROPINA 5 mg/ml
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar por debajo de 25ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el frasco y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Para evitar infecciones, debe desechar el frasco 4 semanas después de haberlo abierto por primera vez.
Anote la fecha de apertura del frasco en el recuadro reservado para esta finalidad en la caja.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de COLIROFTA ATROPINA 5 mg/ml
- El principio activo es atropina. Un ml de colirio en solución contiene 5 mg de atropina (como sulfato) (0,5%).
- Los demás componentes son: parahidroxibenzoato de metilo (E-218), parahidroxibenzoato de propilo (E-216), cloruro de sodio, hidrogenofosfato de sodio dodecahidrato, dihidrogenofosfato de potasio y agua purificada.
Aspecto del producto y contenido del envase
Colirofta Atropina 5 mg/ml es un colirio en solución; es un líquido (solución trasparente e incolora) que se presenta en un envase cuentagotas (frasco de plástico) de 10 ml con tapón precinto.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Alcon Healthcare S.A.
World Trade Center Almeda Park
Plaça de la Pau s/n, Edificio 6, planta 3
08940 - Cornellà de Llobregat (Barcelona)
Spain
Responsable de la fabricación
Siegfried El Masnou, S.A.
C/ Camil Fabra, 58
08320 El Masnou – Barcelona
Spain
o
Alcon Laboratories Belgium
Lichterveld 3
2870 Puurs-Sint-Amands
Belgium
Fecha de la última revisión de este prospecto:Mayo 2020
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Precio medio en farmacia5.1 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a COLIROFTA ATROPINA 5 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, -Principio activo: atropineFabricante: Alcon Healthcare S.A.Requiere recetaForma farmacéutica: COLIRIO, 10 mgPrincipio activo: cyclopentolateFabricante: Alcon Healthcare S.A.Requiere recetaForma farmacéutica: COLIRIO, 10 mg/mlPrincipio activo: TropicamidaFabricante: Alcon Healthcare S.A.Requiere receta
Médicos online para COLIROFTA ATROPINA 5 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de COLIROFTA ATROPINA 5 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















