ESPEROCT 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar ESPEROCT 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Esperoct 500UI polvo y disolvente para solución inyectable
Esperoct 1000UI polvo y disolvente para solución inyectable
Esperoct 1500UI polvo y disolvente para solución inyectable
Esperoct 2000UI polvo y disolvente para solución inyectable
Esperoct 3000UI polvo y disolvente para solución inyectable
Esperoct 4000UI polvo y disolvente para solución inyectable
Esperoct 5000UI polvo y disolvente para solución inyectable
turoctocog alfa pegol [factor VIII de coagulación humano pegilado (ADNr)]
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Esperoct y para qué se utiliza
- Qué necesita saber antes de empezar a usar Esperoct
- Cómo usar Esperoct
- Posibles efectos adversos
- Conservación de Esperoct
- Contenido del envase e información adicional
1. Qué es Esperoct y para qué se utiliza
Qué es Esperoct
Esperoct contiene el principio activo turoctocog alfa pegol y es un medicamento de factor VIII de coagulación recombinante de acción prolongada. El factor VIII es una proteína que se encuentra en la sangre y que ayuda a prevenir y detener el sangrado.
Para qué se utiliza Esperoct
Esperoct se utiliza para tratar y prevenir sangrados en personas de todos los grupos de edad con hemofilia A (deficiencia congénita de factor VIII).
En personas con hemofilia A, el factor VIII falta o no funciona correctamente. Esperoct sustituye este factor VIII ausente o defectuoso y ayuda a que la sangre forme coágulos en el lugar del sangrado.
2. Qué necesita saber antes de empezar a usar Esperoct
No use Esperoct
- si es alérgico a turoctocog alfa pegol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- si es alérgico a las proteínas de hámster.
No use Esperoct si se encuentra en alguna de las situaciones anteriores. Si no está seguro de ello, consulte a su médico antes de usar este medicamento.
Advertencias y precauciones
Uso previo de medicamentos con factorVIII
Informe a su médico si ha usado con anterioridad medicamentos con factor VIII, especialmente si ha desarrollado inhibidores (anticuerpos) contra el medicamento, ya que puede haber riesgo de que esto vuelva a suceder.
Reacciones alérgicas
Existe riesgo de que se pueda producir una reacción alérgica grave y repentina (p. ej., una reacción anafiláctica) a Esperoct.
Si presenta signos tempranos de reacciones alérgicas, detenga la inyección y póngase en contacto con su médico o con el servicio de urgencias de inmediato. Estos signos tempranos pueden ser erupción, sarpullido, ronchas, picor en amplias zonas de la piel, enrojecimiento y/o hinchazón de los labios, la lengua, la cara o las manos, dificultad para tragar o respirar, sibilancias, opresión en el pecho, piel pálida y fría, palpitaciones, o mareo, dolor de cabeza, náuseas y vómitos.
Desarrollo de “inhibidores del factorVIII” (anticuerpos)
Se pueden desarrollar inhibidores (anticuerpos) durante el tratamiento con todos los medicamentos con factor VIII
- Estos inhibidores, especialmente a niveles altos, impiden que el tratamiento funcione correctamente
- Se le vigilará detenidamente por si presenta desarrollo de inhibidores
- Si su hemorragia no se está controlando con Esperoct, informe a su médico inmediatamente
- No aumente la dosis total de Esperoct para controlar el sangrado sin hablar con su médico.
Complicaciones relacionadas con el catéter
Si tiene un catéter por el que se le inyectan medicamentos en la sangre (un dispositivo de acceso venoso central), podría desarrollar infecciones o coágulos de sangre en el lugar de inserción del catéter.
Enfermedad del corazón
Hable con su médico o farmacéutico si tiene una enfermedad cardíaca o si tiene riesgo de padecer una enfermedad cardíaca.
Otros medicamentos y Esperoct
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
La influencia de Esperoct sobre la capacidad para conducir y utilizar máquinas es nula.
Disminución de la actividad del factorVIII en pacientes previamente no tratados
Puede producirse una disminución de la actividad del factor VIII al inicio de su tratamiento. Si su sangrado no se está controlando con Esperoct, informe a su médico.
Disminución de la actividad del factorVIII en pacientes previamente tratados
Puede producirse una disminución de la actividad de factor VIII al inicio de su tratamiento. Informe a su médico si su dosis habitual de Esperoct no está controlando su sangrado.
Esperoct contiene sodio
Este medicamento contiene 30,5 mg de sodio (componente principal de la sal de mesa/para cocinar) por vial reconstituido. Equivalente al 1,5% de la ingesta diaria máxima de sodio recomendada para un adulto.
3. Cómo usar Esperoct
Un médico con experiencia en el tratamiento de personas con hemofilia A iniciará el tratamiento con Esperoct.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda sobre cómo usar Esperoct, consulte de nuevo a su médico.
Cómo se administra Esperoct
Esperoct se administra mediante inyección en una vena (vía intravenosa), ver “Instrucciones para utilizar Esperoct” para obtener más información.
Cuánto usar
Su médico calculará su dosis en función de su peso corporal y de si se usa para prevenir o para tratar un sangrado.
Para prevenir sangrados
Para niños (menores de 12 años de edad), la dosis recomendada es de 65 UI de Esperoct por kg de peso corporal dos veces a la semana. Su médico puede elegir otra dosis o la frecuencia con la que se deben administrar las inyecciones, de acuerdo a sus necesidades.
Para adultos y adolescentes (12 años de edad o más), la dosis recomendada es 50 UI de Esperoct por kg de peso corporal cada 4 días. Su médico puede elegir otra dosis o la frecuencia con la que se deben administrar las inyecciones, de acuerdo a sus necesidades.
Para tratar sangrados
La dosis de Esperoct se calcula en función de su peso corporal y de los niveles de factor VIII que se deseen alcanzar. El valor deseado de niveles de factor VIII depende de la gravedad y la localización del sangrado. Informe a su médico si su dosis habitual de Esperoct no está controlando su sangrado.
Uso en niños y adolescentes
Para niños (menores de 12 años de edad), la dosis recomendada es de 65 UI de Esperoct por kg de peso corporal dos veces a la semana. Los adolescentes (12 años de edad o más) pueden usar la misma dosis que los adultos.
Si usa más Esperoct del que debe
Si usa más Esperoct del que debe, contacte con su médico inmediatamente.
Use siempre Esperoct tal y como su médico le haya indicado. Si no está seguro, consulte a su médico. Para más información, ver “Desarrollo de inhibidores del factor VIII (anticuerpos)” en la sección 2.
Si olvidó usar Esperoct
Si olvidó una dosis, inyéctese la dosis omitida en cuanto se acuerde. No se inyecte una dosis doble para compensar la dosis olvidada. Continúe con la siguiente inyección como estaba programada y siga los consejos de su médico. Si tiene alguna duda, contacte con su médico.
Si interrumpe el tratamiento con Esperoct
No interrumpa el tratamiento con Esperoct sin hablar antes con su médico.
Si interrumpe el tratamiento con Esperoct dejará de estar protegido frente al sangrado o es posible que un sangrado ya existente no se detenga. Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas (hipersensibilidad)
Detenga la inyección inmediatamente si desarrolla reacciones alérgicas graves y repentinas (reacciones anafilácticas). Si presenta alguno de los siguientes síntomas de una reacción alérgica, póngase en contacto con su médico o con el servicio de urgencias inmediatamente:
- dificultad para tragar o respirar
- sibilancias
- opresión en el pecho
- enrojecimiento y/o hinchazón de los labios, la lengua, la cara o las manos
- erupciones, sarpullidos, ronchas o picor
- piel pálida y fría, palpitaciones o mareos (presión sanguínea baja)
- dolor de cabeza, sentirse enfermo (náuseas) o estar enfermo (vómitos).
Desarrollo de “inhibidores del factorVIII” (anticuerpos)
Si ha recibido previamente más de 150 días de tratamiento con factor VIII, puede que desarrolle inhibidores (anticuerpos) (puede afectar hasta a 1 de cada 100 personas). Si esto le sucede, el tratamiento puede dejar de actuar adecuadamente y puede sufrir un sangrado persistente. Si esto sucede, póngase en contacto con su médico inmediatamente. Ver “Desarrollo de inhibidores del factor VIII (anticuerpos)” en la sección 2.
Se han observado los siguientes efectos adversos con Esperoct
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- inhibidores del factor VIII (anticuerpos) en pacientes que no han sido tratados previamente con factor VIII.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- reacciones en la piel en el lugar de la inyección
- picor (prurito)
- enrojecimiento de la piel (eritema)
- sarpullido.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- reacciones alérgicas (hipersensibilidad). Estas pueden llegar a ser graves y poner en peligro la vida, ver la sección anterior “Reacciones alérgicas (hipersensibilidad)” para más información
- inhibidores del factor VIII (anticuerpos) en pacientes tratados previamente con factor VIII.
Otros efectos adversos posibles(frecuencia no conocida)
Disminución de la actividad del factor VIII en ausencia de inhibidores del factor VIII.
Puede producirse una respuesta temporal del sistema inmunitario al inicio del tratamiento, lo que puede reducir la eficacia del medicamento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Esperoct
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en las etiquetas del vial y de la jeringa precargada después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Antes de la reconstitución(antes de mezclar el polvo con el disolvente):
Conservar en nevera (entre 2 °C y 8 °C). Esperoct se puede conservar
- a temperatura ambiente (≤ 30 °C) durante un periodo único no superior a 1 año durante el periodo de validez del medicamento o
- por encima de la temperatura ambiente (> 30 °C hasta 40 °C) durante un periodo único no superior a 3 meses durante el periodo de validez del medicamento.
Cuando comience a conservar Esperoct fuera de nevera, anote la fecha y la temperatura de almacenamiento en el lugar provisto en el envase de cartón.
Una vez que ha sacado el medicamento de la nevera para almacenarlo no puede volver a meterlo en la nevera. No congelar. Conservar en el embalaje original para protegerlo de la luz.
Después de la reconstitución(después de haber mezclado el polvo con el disolvente – 500 UI, 1 000 UI, 1 500 UI, 2 000 UI, 3 000 UI):
Una vez que se ha reconstituido Esperoct, se debe usar de inmediato. Si no puede utilizar la solución reconstituida inmediatamente, se debe usar en
- 24 horas cuando se conserva en nevera (entre 2 °C y 8 °C) o
- 4 horas a ≤ 30 °C o
- 1 hora entre > 30 °C y 40 °C, solo si el producto se ha conservado antes de la reconstitución por encima de temperatura ambiente (> 30 °C hasta 40 °C) no más de 3 meses.
Después de la reconstitución(después de haber mezclado el polvo con el disolvente – 4 000 UI, 5 000 UI):
Se ha demostrado la estabilidad química y física en uso para:
- 24 horas cuando se conserva en nevera (entre 2 °C y 8 °C) o
- 4 horas a ≤ 30 °C.
El polvo del vial es un polvo de blanco a blanquecino. Si el color del polvo ha cambiado, no lo utilice.
La solución reconstituida debe ser transparente e incolora. No utilice la solución reconstituida si observa que contiene partículas o decoloración.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Esperoct
- El principio activo es turoctocog alfa pegol [factor VIII de coagulación humano pegilado (ADNr)]. Cada vial de Esperoct contiene 500, 1 000, 1 500, 2 000, 3 000, 4 000 o 5 000 UI de turoctocog alfa pegol.
- Los demás componentes son L‑histidina, sacarosa, polisorbato 80, cloruro de sodio, L‑metionina, cloruro de calcio dihidratado, hidróxido de sodio y ácido clorhídrico.
- Los componentes del disolvente son solución inyectable de cloruro de sodio 9 mg/ml (0,9%) y agua para preparaciones inyectables.
Ver sección 2 “Esperoct contiene sodio”.
Después de la reconstitución con el disolvente suministrado [solución inyectable de cloruro de sodio 9 mg/ml (0,9%)], la solución inyectable preparada contiene 125, 250, 375, 500, 750, 1 000 o 1 250 UI de turoctocog alfa pegol por ml, respectivamente (basado en la concentración de turoctocog alfa pegol, eso es, 500, 1 000, 1 500, 2 000, 3 000, 4 000 o 5 000 UI).
Aspecto de Esperoct y contenido del envase
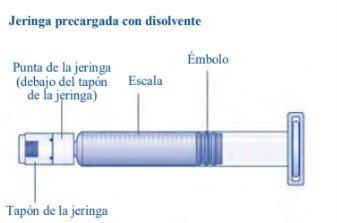
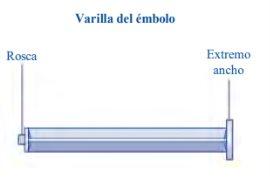
Esperoct está disponible en envases que contienen 500 UI, 1 000 UI, 1 500 UI, 2 000 UI, 3 000 UI, 4 000 UI o 5 000 UI. Cada envase de Esperoct contiene un vial con polvo de color blanco a blanquecino, una jeringa precargada con 4 ml de un disolvente incoloro y transparente, una varilla de émbolo y un adaptador de vial.
Titular de la autorización de comercialización y responsable de la fabricación
Novo Nordisk A/S
Novo Allé
DK‑2880 Bagsværd, Dinamarca
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
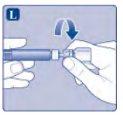
Instrucciones de uso Esperoct Lea atentamente estas instrucciones antes de usar Esperoct. Esperoct se suministra en forma de polvo. Antes de la inyección, se debe reconstituir con el disolvente suministrado en la jeringa. El disolvente es una solución inyectable de cloruro de sodio 9 mg/ml (0,9%). El medicamento reconstituido se debe inyectar en una vena [inyección intravenosa (IV)]. Los elementos de este envase están diseñados para reconstituir e inyectar Esperoct. También necesitará:
Estos elementos no se incluyen en el envase de Esperoct. No utilice el equipo sin haber recibido la formación adecuada de su médico o enfermero. Lávese siempre las manos y asegúrese de que el área a su alrededor esté limpia. Al preparar e inyectar el medicamento directamente en una vena, es importante usar una técnica limpia y sin gérmenes (aséptica).Una técnica incorrecta puede introducir gérmenes capaces de infectar la sangre. No abra el equipo hasta que esté listo para usarlo. No utilice el equipo si se ha caído o se ha dañado.Utilice un envase nuevo en su lugar. No utilice el equipo si ha caducado.Utilice un envase nuevo en su lugar. La fecha de caducidad está impresa en el embalaje exterior, el vial, el adaptador del vial y la jeringa precargada. No utilice el equipo si sospecha que está contaminado.Utilice un envase nuevo en su lugar. No deseche ningún elemento hasta que se haya inyectado la solución reconstituida. El equipo es de un solo uso. | |
Contenido El envase contiene:
| |
| |
No utilice ningún otro sistema para calentarel vial y la jeringa precargada. |
|
|
|
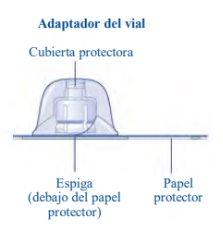
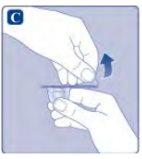
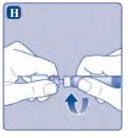
Si el papel protector no está totalmente sellado o si está roto, no utilice el adaptador del vial. No saque el adaptador del vial de la cubierta protectora con los dedos. Si toca la espiga del adaptador del vial, puede transferir gérmenes de sus dedos. |
|
Una vez unido, no retire del vial el adaptador del vial. |
|
No quite el adaptador del vialal quitar la cubierta protectora. |
|
|
|
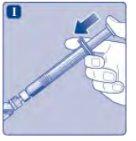
No toque la punta de la jeringa debajo del tapón de la jeringa.Si toca la punta de la jeringa, le puede transferir gérmenes de sus dedos. Si el capuchón de la jeringa está flojo o falta, no utilice la jeringa precargada. |
|
|
|
|
|
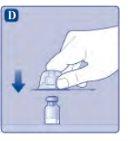
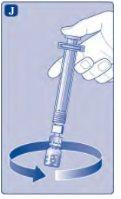
No agite el vial, ya que esto produciría espuma.
|
|
Se recomienda usar Esperoct inmediatamente después de reconstituirlo. Si no puede usar la solución de Esperoct reconstituida inmediatamente (aplica a 500UI, 1000UI, 1500UI, 2000UI, 3000UI),debe usarla en un plazo de:
Si no puede usar la solución de Esperoct reconstituida inmediatamente (aplica a 4000UI, 5000UI),debe usarla en un plazo de:
Conserve el medicamento reconstituido en el vial. No congele la solución reconstituida ni la guarde en jeringas. Guardela solución reconstituida alejada de la luz directa. Si su dosis requiere más de un vial, repita los pasos Ahasta Jcon más viales, adaptadores de vial y jeringas precargadas hasta alcanzar la dosis necesaria. | |
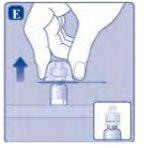
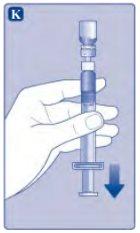
Si en algún momento hay aire en la jeringa, inyecte el aire de nuevo en el vial.
|
|
No toque la punta de la jeringa. Si toca la punta de la jeringa, le puede transferir gérmenes de sus dedos. |
|
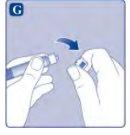
Ahora Esperoct está listo para ser inyectado en una vena.
No mezcle Esperoct con otras inyecciones o medicamentos intravenosos. Inyección de Esperoct a través de conectores sin aguja para catéteres intravenosos (IV) Precaución:La jeringa precargada es de cristal y está diseñada para ser compatible con conexiones luer‑lock estándar. Algunos conectores sin aguja que presentan una espiga interna son incompatibles con la jeringa precargada. Esta incompatibilidad puede impedir la administración del medicamento y originar un daño del conector sin aguja. Inyección de la solución a través de un dispositivo de acceso venoso central (DAVC) como un catéter venoso central o un puerto subcutáneo:
| |
Eliminación
No los tire a la basura doméstica. |
|
No desmonte el equipo antes de eliminarlo. No reutilice el equipo. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ESPEROCT 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1.000 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1.500 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1000 UI - tras reconstitución en 2 ml de agua para inyectables la dosis es de 500 UI/mlPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para ESPEROCT 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ESPEROCT 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes