Elocta 3000 UI polvo y disolvente para solucion inyectable

Cómo usar Elocta 3000 UI polvo y disolvente para solucion inyectable
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ELOCTA 250 UI polvo y disolvente para solución inyectable
ELOCTA 500 UI polvo y disolvente para solución inyectable
ELOCTA 750 UI polvo y disolvente para solución inyectable
ELOCTA 1000 UI polvo y disolvente para solución inyectable
ELOCTA 1500 UI polvo y disolvente para solución inyectable
ELOCTA 2000 UI polvo y disolvente para solución inyectable
ELOCTA 3000 UI polvo y disolvente para solución inyectable
ELOCTA 4000 UI polvo y disolvente para solución inyectable
efmoroctocog alfa (factor VIII de coagulación recombinante)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es ELOCTA y para qué se utiliza
- Qué necesita saber antes de empezar a usar ELOCTA
- Cómo usar ELOCTA
- Posibles efectos adversos
- Conservación de ELOCTA
- Contenido del envase e información adicional
1. Qué es ELOCTA y para qué se utiliza
ELOCTA contiene el principio activo efmoroctocog alfa, un factor VIII de coagulación recombinante, proteína de fusión Fc. El factor VIII es una proteína producida de forma natural por el cuerpo y es necesaria para que la sangre forme coágulos y detener las hemorragias. ELOCTA es un medicamento utilizado para el tratamiento y la prevención de las hemorragias en los pacientes de todos los grupos de edad con hemofilia A (un trastorno hemorrágico hereditario causado por una deficiencia del factor VIII).
ELOCTA se prepara mediante tecnología recombinante sin la adición de ningún componente de origen humano o animal en el proceso de fabricación.
Como actúa ELOCTA
En los pacientes con hemofilia A, el factor VIII está ausente o no funciona adecuadamente. ELOCTA se utiliza para sustituir el factor VIII ausente o deficiente. ELOCTA aumenta las concentraciones de factor VIII en la sangre y corrige temporalmente la tendencia a sufrir hemorragias.
2. Qué necesita saber antes de empezar a usar ELOCTA
No use ELOCTA:
- si es alérgico al efmoroctocog alfa o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar ELOCTA.
- Existe una pequeña posibilidad de que sufra una reacción anafiláctica (una reacción alérgica grave y repentina) a ELOCTA. Entre los signos de las reacciones alérgicas se encuentran picor generalizado, ronchas, sensación de opresión en el pecho, dificultad para respirar y presión arterial baja. Si aparece cualquiera de estos síntomas, interrumpa inmediatamente la inyección y póngase en contacto con su médico.
- La formación de inhibidores (anticuerpos) es una complicación conocida que puede producirse durante el tratamiento con todos los medicamentos compuestos por factor VIII. Estos inhibidores, especialmente en grandes cantidades, impiden que el tratamiento funcione correctamente, por lo que se les supervisará cuidadosamente a usted y a su hijo por si desarrollan dichos inhibidores. Si su hemorragia o la de su hijo no se está controlando con ELOCTA, consulte a su médico inmediatamente.
Acontecimientos cardiovasculares
Si tiene una enfermedad del corazón o se encuentra en riesgo de sufrirla, tenga especial cuidado al utilizar medicamentos con factor VIII y consulte a su médico.
Complicaciones relacionadas con el catéter
Si necesita un dispositivo de acceso venoso central (DAVC), se debe tener en cuenta el riesgo de complicaciones relacionadas con el DAVC, incluidas las infecciones locales, la presencia de bacterias en la sangre y la trombosis en el lugar de inserción del catéter.
Documentación
Le recomendamos encarecidamente que cada vez que se administre ELOCTA, se anoten el nombre y el número de lote del producto.
Otros medicamentos y ELOCTA
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se han observado efectos sobre la capacidad para conducir o utilizar máquinas.
ELOCTA contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por vial; esto es, esencialmente “exento de sodio”.
No obstante, dependiendo de su peso corporal y de la dosis, podría recibir más de un vial, lo que debe tenerse en cuenta si sigue una dieta pobre en sodio.
3. Cómo usar ELOCTA
El tratamiento con ELOCTA lo iniciará un médico con experiencia en el cuidado de pacientes con hemofilia. Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico (ver sección Instrucciones de preparación y administración). En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
ELOCTA se administra mediante inyección en una vena. Su médico calculará su dosis de ELOCTA (en Unidades Internacionales o “UI”), dependiendo de sus necesidades individuales de tratamiento de sustitución del factor VIII y de si se utiliza para la prevención o el tratamiento de las hemorragias. Consulte a su médico si cree que no está consiguiendo controlar las hemorragias con la dosis que recibe.
Con qué frecuencia necesitará una inyección, dependerá del grado de eficacia que ELOCTA esté mostrando con usted. Su médico le realizará las pruebas de laboratorio pertinentes para asegurarse de que tiene concentraciones adecuadas de factor VIII en la sangre.
Tratamiento de las hemorragias
La dosis de ELOCTA se calcula en función de su peso corporal y de las concentraciones de factor VIII que se desean conseguir. Las concentraciones objetivo de factor VIII dependen de la gravedad y la localización de la hemorragia.
Prevención de las hemorragias
La dosis habitual de ELOCTA es de 50 UI por kg de peso corporal, administradas cada 3 a 5 días. Su médico puede ajustar la dosis en un intervalo comprendido entre 25 y 65 UI por kg de peso corporal. En algunos casos, especialmente en los pacientes más jóvenes, puede ser necesario usar intervalos de dosificación más cortos o dosis mayores.
Uso en niños y adolescentes
ELOCTA se puede utilizar en niños y adolescentes de todas las edades. En los niños menores de 12 años, pueden ser necesarias dosis más altas o inyecciones más frecuentes.
Si usa más ELOCTA del que debe
Informe a su médico lo antes posible. Siga exactamente las instrucciones de administración de ELOCTA indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Si olvidó usar ELOCTA
No tome una dosis doble para compensar las dosis olvidadas. Tome su dosis tan pronto se acuerde y después reanude su pauta normal de dosificación. Si no está seguro de lo que debe hacer, consulte a su médico o farmacéutico.
Si interrumpe el tratamiento con ELOCTA
No interrumpa el tratamiento con ELOCTA sin consultar a su médico. Si interrumpe el tratamiento con ELOCTA, es posible que ya no esté protegido contra las hemorragias o que una hemorragia ya existente no se detenga.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si se producen reacciones alérgicas graves y repentinas (reacción anafiláctica), la inyección se debe interrumpir inmediatamente. Se debe poner en contacto con su médico de inmediato si presenta alguno de los siguientes síntomas de las reacciones alérgicas: hinchazón de la cara, erupción, picor generalizado, ronchas, sensación de opresión en el pecho, dificultad para respirar, escozor y pinchazos en el lugar de la inyección, escalofríos, sofocos, dolor de cabeza, presión arterial baja, sensación general de malestar, náuseas, agitación y latido cardiaco rápido, sensación de mareo o pérdida del conocimiento.
En los niños sin tratamiento previo con medicamentos compuestos por factor VIII pueden producirse anticuerpos inhibidores (ver sección 2) muy frecuentemente (más de 1 de cada 10 pacientes); sin embargo, en los pacientes que han recibido tratamiento previo con factor VIII (más de 150 días de tratamiento), el riesgo es poco frecuente (menos de 1 de cada 100 pacientes). Si esto sucede, los medicamentos pueden dejar de funcionar correctamente y usted puede sufrir una hemorragia persistente. En ese caso, contacte con su médico inmediatamente.
Con este medicamento pueden aparecer los siguientes efectos adversos.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
Dolor de cabeza, mareo, alteraciones del gusto, latido cardiaco lento, presión arterial alta, sofocos, dolor vascular después de la inyección, tos, dolor abdominal bajo, erupción cutánea, erupción papular, trombosis relacionada con el dispositivo, hinchazón articular, dolor muscular, dolor de espalda, dolor articular, malestar general, dolor en el pecho, sensación de frío, sensación de calor y presión arterial baja.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ELOCTA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta del vial después de “CAD/EXP”. La fecha de caducidad es el último día del mes que se indica. No utilice este medicamento si se ha conservado a temperatura ambiente durante más de 6 meses.
Conservar en nevera (entre 2 °C - 8 °C).
No congelar.
Conservar en el embalaje original para protegerlo de la luz.
Alternativamente, ELOCTA se puede conservar a temperatura ambiente (hasta 30 °C) durante un periodo único que no supere los 6 meses. Anote en la caja la fecha en la que se extrajo ELOCTA de la nevera y se dejó a temperatura ambiente. Tras la conservación a temperatura ambiente, el medicamento no se debe volver a introducir en la nevera.
Una vez haya preparado ELOCTA, debe utilizarlo inmediatamente. Si no puede usar la solución preparada de ELOCTA de inmediato, debe utilizarla en un plazo máximo de 6 horas. No refrigere la solución preparada. Proteja la solución preparada de la luz solar directa.
La solución preparada debe ser transparente a ligeramente opalescente e incolora. No utilice este medicamento si observa que está turbio o contiene partículas visibles.
Elimine adecuadamente cualquier resto de solución no utilizada. Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ELOCTA
- El principio activo es efmoroctocog alfa (factor VIII de coagulación recombinante, proteína de fusión Fc). Cada vial de ELOCTA contiene nominalmente 250, 500, 750, 1.000, 1.500, 2.000, 3.000 o 4.000 UI de efmoroctocog alfa.
- Los demás componentes son sacarosa, cloruro de sodio, L-histidina, cloruro de calcio dihidrato, polisorbato 20, hidróxido de sodio, ácido clorhídrico y agua para preparaciones inyectables. Ver sección 2 si sigue una dieta pobre en sodio.
Aspecto del producto y contenido del envase
ELOCTA se presenta en forma de polvo y disolvente para solución inyectable. El polvo es un polvo o torta de color blanco a blanquecino. El disolvente suministrado para la preparación de la solución inyectable es una disolución transparente e incolora. Tras la preparación, la solución para inyectar es de transparente a ligeramente opalescente e incolora.
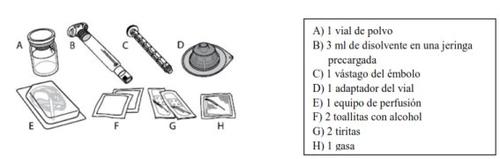
Cada envase de ELOCTA contiene 1 vial de polvo, 3 ml de disolvente en una jeringa precargada, 1 vástago del émbolo, 1 adaptador del vial, 1 equipo de perfusión, 2 toallitas con alcohol, 2 tiritas y 1 gasa.
Titular de la autorización de comercialización y responsable de la fabricación
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm,
Suecia
Fecha de la última revisión de este prospecto: 01/2021
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Dé la vuelta al prospecto para consultar las instrucciones de preparación y administración
Instrucciones de preparación y administración
ELOCTA se administra mediante inyección intravenosa (IV) después de disolver el polvo inyectable con el disolvente suministrado en la jeringa precargada. El envase de ELOCTA contiene:
ELOCTA no debe mezclarse con otras soluciones inyectables o para perfusión.
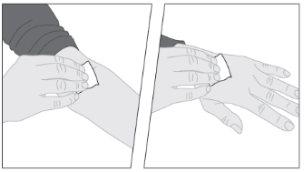
Lávese las manos antes de abrir el envase.
Preparación:
|
|
|
|
|
|
|
|
|
|
|
No lo agite. |
|
|
|
Nota: si usa más de un vial de ELOCTA por inyección, cada vial se debe preparar por separado conforme a las instrucciones previas (pasos 1 a 13) y la jeringa de disolvente se debe retirar, dejando el adaptador del vial colocado en su posición. Se puede utilizar una única jeringa luer lock más grande para extraer el contenido preparado de cada uno de los viales. |
Nota: si la solución no se va a utilizar inmediatamente, la cápsula de cierre de la jeringa se debe volver a colocar cuidadosamente sobre la punta de la jeringa. No toque la punta de la jeringa ni el interior de la cápsula de cierre. Tras la preparación, ELOCTA se puede conservar a temperatura ambiente durante un máximo de 6 horas antes de la administración. Una vez transcurrido este tiempo, la solución preparada de ELOCTA se debe desechar. Protéjala de la luz solar directa. |
Administración (inyección intravenosa):
ELOCTA se debe administrar utilizando el equipo de perfusión (E) suministrado en el envase.
|
|
|
|
|
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a Elocta 3000 UI polvo y disolvente para solucion inyectableForma farmacéutica: INYECTABLE, 1.000 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1.500 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1000 UI - tras reconstitución en 2 ml de agua para inyectables la dosis es de 500 UI/mlPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para Elocta 3000 UI polvo y disolvente para solucion inyectable
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de Elocta 3000 UI polvo y disolvente para solucion inyectable, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes





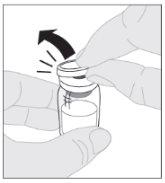 Coloque el vial sobre una superficie limpia y plana. Retire la cápsula de cierre de plástico del vial de ELOCTA.
Coloque el vial sobre una superficie limpia y plana. Retire la cápsula de cierre de plástico del vial de ELOCTA.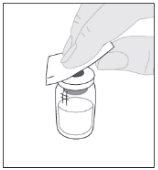 Limpie la parte superior del vial con una de las toallitas con alcohol (F) suministradas en el envase y deje que se seque al aire. No toque la parte superior del vial ni permita que entre en contacto con nada una vez la haya limpiado.
Limpie la parte superior del vial con una de las toallitas con alcohol (F) suministradas en el envase y deje que se seque al aire. No toque la parte superior del vial ni permita que entre en contacto con nada una vez la haya limpiado. 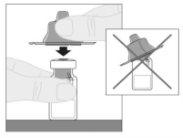
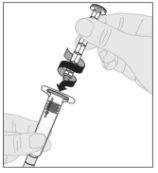 Acople el vástago del émbolo (C) a la jeringa de disolvente insertando la punta del vástago en la apertura del émbolo de la jeringa. Gire el vástago del émbolo firmemente en el sentido de las agujas del reloj hasta que quede bien asentado en el émbolo de la jeringa.
Acople el vástago del émbolo (C) a la jeringa de disolvente insertando la punta del vástago en la apertura del émbolo de la jeringa. Gire el vástago del émbolo firmemente en el sentido de las agujas del reloj hasta que quede bien asentado en el émbolo de la jeringa.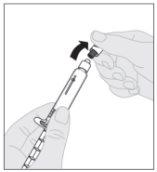 Desprenda la cápsula de cierre de seguridad inviolable de plástico blanco de la jeringa de disolvente doblándola por la cápsula de cierre de perforación hasta que se rompa. Deje la cápsula de cierre aparte colocándola con la parte de arriba mirando hacia abajo sobre una superficie plana. No toque el interior de la cápsula de cierre ni la punta de la jeringa.
Desprenda la cápsula de cierre de seguridad inviolable de plástico blanco de la jeringa de disolvente doblándola por la cápsula de cierre de perforación hasta que se rompa. Deje la cápsula de cierre aparte colocándola con la parte de arriba mirando hacia abajo sobre una superficie plana. No toque el interior de la cápsula de cierre ni la punta de la jeringa.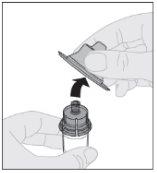
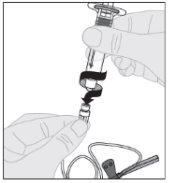
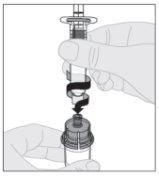 Conecte la jeringa de disolvente al adaptador del vial insertando la punta de la jeringa en la apertura del adaptador. Empuje firmemente y gire la jeringa en el sentido de las agujas del reloj hasta que quede bien conectada.
Conecte la jeringa de disolvente al adaptador del vial insertando la punta de la jeringa en la apertura del adaptador. Empuje firmemente y gire la jeringa en el sentido de las agujas del reloj hasta que quede bien conectada.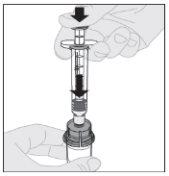 Presione lentamente hacia abajo el vástago del émbolo para inyectar todo el disolvente en el vial de ELOCTA.
Presione lentamente hacia abajo el vástago del émbolo para inyectar todo el disolvente en el vial de ELOCTA.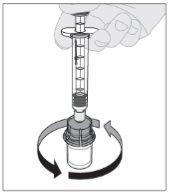
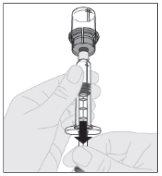 Asegurándose de que el vástago del émbolo de la jeringa siga completamente presionado hacia abajo, invierta el vial. Tire lentamente del vástago del émbolo para trasladar toda la solución al interior de la jeringa a través del adaptador del vial.
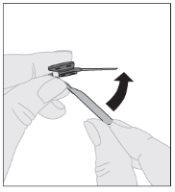
Asegurándose de que el vástago del émbolo de la jeringa siga completamente presionado hacia abajo, invierta el vial. Tire lentamente del vástago del émbolo para trasladar toda la solución al interior de la jeringa a través del adaptador del vial.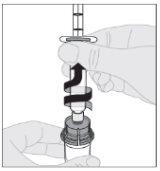 Desacople la jeringa del adaptador del vial tirando suavemente del vial al tiempo que lo gira en el sentido contrario a las agujas del reloj.
Desacople la jeringa del adaptador del vial tirando suavemente del vial al tiempo que lo gira en el sentido contrario a las agujas del reloj.