
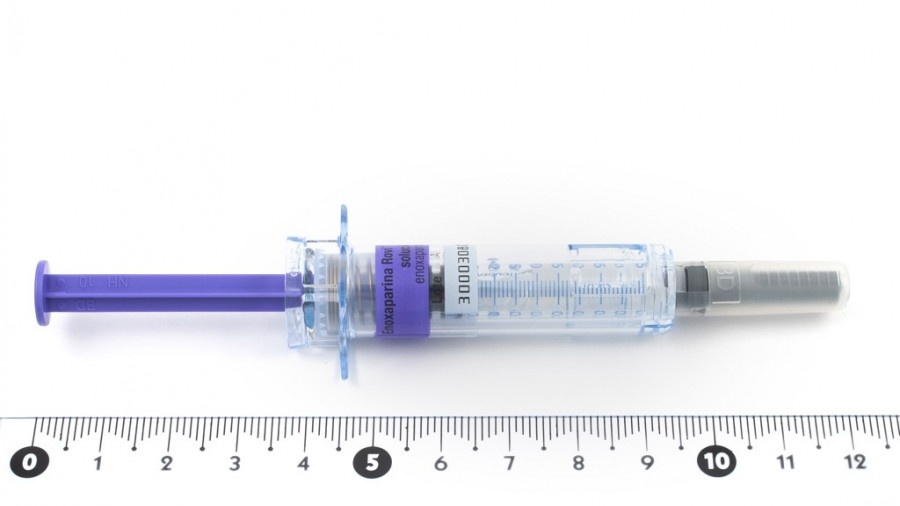
CLEXANE 12.000 UI (120 mg)/ 0,8 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar CLEXANE 12.000 UI (120 mg)/ 0,8 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Clexane y para qué se utiliza
- Qué necesita saber antes de empezar a usar Clexane
- No use Clexane si le afecta cualquiera de las condiciones mencionadas. Si no está seguro, consulte a su médico o farmacéutico antes de usar Clexane.
- En caso de que le vayan a realizar una punción lumbar o espinal, o vaya a someterse a una operación quirúrgica donde se vaya a utilizar una anestesia espinal o epidural, informe a su médico que está usando Clexane.
- Cómo usar Clexane
- Posibles efectos adversos
- Conservación de Clexane
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
CLEXANE 12.000 UI (120 mg)/0,8 ml solución inyectable en jeringa precargada
enoxaparina sódica
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermera.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Clexane y para qué se utiliza
- Qué necesita saber antes de empezar a usar Clexane
- Cómo usar Clexane
- Posibles efectos adversos
- Conservación de Clexane
- Contenido del envase e información adicional
1. Qué es Clexane y para qué se utiliza
Clexane contiene un principio activo denominado enoxaparina sódica. Pertenece a un grupo de medicamentos denominado “heparina de bajo peso molecular” o HBPM.
Cómo actúa Clexane
Clexane actúa de dos formas:
- Impidiendo que los coágulos de sangre ya existentes se hagan más grandes. Esto ayuda a su organismo a romperlos y que no sigan haciéndole daño.
- Interrumpiendo la formación de nuevos coágulos en la sangre.
Para qué se usa Clexane
Se puede usar Clexane para:
- Tratar los coágulos que hay en sangre
- Evitar la formación de coágulos en sangre en las siguientes situaciones:
- antes y después de una operación quirúrgica
- cuando tenga una enfermedad a corto plazo y no pueda moverse durante algún tiempo
- si ha tenido un coágulo de sangre debido al cáncer, para prevenir que se formen más coágulos.
- Para evitar la formación de coágulos de sangre cuando tenga angina inestable (una enfermedad en la que no llega suficiente cantidad de sangre al corazón) o después de un ataque al corazón
- Evitar la formación de coágulos de sangre en los tubos del aparato de diálisis (que se emplea en personas que padecen problemas graves de riñón).
2. Qué necesita saber antes de empezar a usar Clexane
No use Clexanesi:
- es alérgico a:
- enoxaparina sódica o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- la heparina o a otras heparinas de bajo peso molecular como nadroparina, tinzaparina o dalteparina.
Los signos de una reacción alérgica incluyen: erupción, problemas para respirar o tragar, hinchazón de cara, labios, lengua, cavidad bucal, garganta u ojos.
- ha tenido una reacción a la heparina que causó una disminución grave en el número de las células que intervienen en la coagulación (plaquetas) en los últimos 100 días
- tiene en sangre anticuerpos frente a la enoxaparina
- está sangrando abundantemente o padece enfermedades de alto riesgo de sangrado como:
- úlcera de estómago, intervención reciente de cerebro u ojos, o hemorragia cerebral.
- está usando Clexane para tratar coágulos en la sangre, y va a tener en 24 horas:
- una punción lumbar o espinal
- una operación con anestesia espinal o epidural.
No use Clexane si le afecta cualquiera de las condiciones mencionadas. Si no está seguro, consulte a su médico o farmacéutico antes de usar Clexane.
Advertencias y precauciones
No se debe intercambiar Clexane con otras “heparinas de bajo peso molecular” como nadroparina, tinzaparina o dalteparina. Esto es porque no son exactamente iguales y no tienen la misma actividad ni las mismas instrucciones de uso.
Consulte con su médico o farmacéutico antes de empezar a usar Clexane si:
- alguna vez ha tenido una reacción a la heparina que causó una disminución grave en el número de células que intervienen en la coagulación (plaquetas)
- le han implantado una válvula cardiaca
- tiene endocarditis (una infección del revestimiento interior del corazón)
- tiene antecedentes de úlcera gástrica
- ha tenido recientemente una hemorragia cerebral
- tiene alta la presión sanguínea
- tiene diabetes o problemas en los vasos sanguíneos de los ojos causados por la diabetes (denominado retinopatía diabética)
- ha sido operado recientemente de ojos o cerebro
- es usted una persona de edad avanzada (por encima de los 65 años) y especialmente si es mayor de 75 años
- tiene problemas de riñón
- tiene problemas de hígado
- presenta un peso muy bajo o tiene sobrepeso
- tiene alto los niveles de potasio en sangre (que podría comprobarse con un análisis de sangre)
- actualmente está usando medicamentos que afectan al sangrado (ver sección 2, “Uso de Clexane con otros medicamentos”)
- tiene cualquier problema con su columna o le han sometido a cirugía espinal.
Si le afecta cualquiera de las condiciones mencionadas (o no está seguro), consulte a su médico o farmacéutico antes de usar Clexane.
Para los pacientes que reciben dosis superiores a 210 mg/día, este medicamento contiene más de 24 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada dosis. Esto equivale al 1,2% de la ingesta diaria máxima de sodio recomendada para un adulto.
Análisis y controles
Podría tener que realizarse un análisis de sangre antes de empezar a utilizar este medicamento, y mientras lo esté usando; esto es para comprobar el nivel de las células que intervienen en la coagulación (plaquetas) y los niveles de potasio en sangre.
Niños y adolescentes
No se ha evaluado la eficacia y seguridad de Clexane en niños o adolescentes.
Uso deClexanecon otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
- warfarina – empleada para reducir la coagulación de la sangre
- aspirina (también conocida como ácido acetilsalicílico o AAS), clopidogrel u otros medicamentos usados para interrumpir la formación de coágulos en la sangre (ver sección 3, “Cambio de medicamento anticoagulante”)
- inyección de dextrano – usado como sustitutivo de la sangre
- ibuprofeno, diclofenaco, ketorolaco y otros medicamentos conocidos como antiinflamatorios no esteroideos utilizados para tratar el dolor y la inflamación en artritis y otras enfermedades
- prednisolona, dexametasona y otros medicamentos utilizados para tratar el asma, la artritis reumatoide y otras enfermedades
- medicamentos que aumentan los niveles de potasio en sangre como sales de potasio, medicamentos para eliminar líquidos (diuréticos), y algunos medicamentos para tratar problemas de corazón.
Operaciones quirúrgicas y anestesia
En caso de que le vayan a realizar una punción lumbar o espinal, o vaya a someterse a una operación quirúrgica donde se vaya a utilizar una anestesia espinal o epidural, informe a su médico que está usando Clexane.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Si está embarazada y tiene implantada una válvula cardiaca mecánica, podría tener un riesgo mayor de que se formen coágulos en sangre. Su médico hablará con usted de este tema.
Si está en período de lactancia o planea dar la lactancia, debe consultar a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Clexane no afecta la capacidad para conducir y usar máquinas.
Se recomienda que el profesional sanitario anote el nombre comercial y el número de lote del producto que usted esté usando.
3. Cómo usar Clexane
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Uso del medicamento
- Normalmente su médico o enfermera le administrará Clexane. Esto es porque se tiene que administrar mediante una inyección.
- Clexane generalmente se administra por inyección debajo de la piel (vía subcutánea).
- Clexane se puede administrar por inyección en sus venas (vía intravenosa) después de ciertos tipos de ataques al corazón u operaciones quirúrgicas.
- Clexane se puede añadir al tubo que sale del cuerpo (línea arterial) al comienzo de una sesión de diálisis.
- No administre Clexane en músculo (vía intramuscular).
Qué cantidad se le administrará
- Su médico decidirá la cantidad de Clexane que se le administrará. La cantidad dependerá del motivo por el que se vaya a usar.
- Si tiene algún problema de riñón puede que se le administre una cantidad menor de Clexane.
- Tratamiento de la formación de coágulos en sangre
- La dosis habitual es 150 UI (1,5 mg) por kilogramo de peso corporal una vez al día o 100 UI (1 mg) por kilogramo de peso corporal dos veces al día.
- Su médico decidirá cuánto tiempo recibirá Clexane.
- Interrupción de la formación de coágulos en sangre durante operaciones quirúrgicas o períodos de movilidad limitada debidos a una enfermedad
- La dosis dependerá de la probabilidad que usted tenga de desarrollar un coágulo. Se le administrará 2.000 UI (20 mg) o 4.000 UI (40 mg) de Clexane al día.
- Si le van a operar, le administrarán generalmente la primera inyección 2 o 12 horas antes de la operación.
- Si tiene movilidad reducida por una enfermedad, le administrarán generalmente 4.000 UI (40 mg) de Clexane al día.
- Su médico decidirá cuánto tiempo recibirá Clexane.
- Interrupción de la formación de coágulos cuando tenga angina inestable o después de que haya tenido un ataque al corazón
- Se puede usar Clexane en 2 tipos diferentes de ataques al corazón.
- La cantidad de Clexane que se le administre dependerá de la edad y del tipo de ataque al corazón que haya tenido.
Ataque de corazón tipo IAMSEST (infarto de miocardio sin elevación del segmento ST):
- La dosis habitual es de 100 UI (1 mg) por kilogramo de peso corporal cada 12 horas.
- Por lo general, su médico le dirá que también tome aspirina (ácido acetilsalicílico).
- Su médico decidirá cuánto tiempo recibirá Clexane.
Ataque de corazón tipo IAMCEST (infarto de miocardio con elevación del segmento ST) si es menor de 75 años:
- Se le administrará una inyección inicial intravenosa de 3.000 UI (30 mg) de Clexane.
- A la vez se le administrará una inyección de Clexane debajo de la piel (inyección subcutánea). La dosis habitual es de 100 UI (1 mg) por kilogramo de peso corporal, cada 12 horas.
- Por lo general, su médico le dirá que también tome aspirina (ácido acetilsalicílico).
- Su médico decidirá cuánto tiempo recibirá Clexane.
Ataque de corazón tipo IAMCEST si tiene 75 años o más:
- La dosis habitual es de 75 UI (0,75 mg) por kilogramo de peso corporal, cada 12 horas.
- La cantidad máxima de Clexane administrada en las dos primeras inyecciones es de 7.500 UI (75 mg).
- Su médico decidirá cuánto tiempo recibirá Clexane.
Para pacientes sometidos a una intervención coronaria percutánea (ICP):
- Dependiendo de cuando se le administró la última inyección de Clexane, su médico podría decidir administrarle una dosis adicional de Clexane antes de una intervención ICP. Sería por inyección en vena.
- Interrupción de la formación de coágulos sanguíneos en los tubos del aparato de diálisis
- La dosis habitual es de 100 UI (1 mg) por kilogramo de peso corporal.
- Clexane se añade al tubo que sale del cuerpo (línea arterial) al comienzo de una sesión de diálisis. Esta cantidad suele ser suficiente para una sesión de 4 horas. Sin embargo, es posible que su médico practique una nueva inyección de 50 UI a 100 UI (de 0,5 a 1 mg) por kilogramo de peso corporal, si fuera necesario.
Si va a inyectarse usted mismo Clexane
Si puede administrarse usted mismo Clexane, su médico o enfermera le mostrarán cómo hacerlo. No intente inyectarse usted mismo si no le han enseñado la forma de hacerlo. Si no está seguro qué hacer, consulte inmediatamente a su médico o enfermera. Si la inyección se realiza bajo la piel de forma correcta (lo que se denomina “inyección subcutánea”), esto ayudará a reducir el dolor y el hematoma en el punto de inyección.
Antes de inyectarse usted mismo Clexane
- Prepare lo que vaya a necesitar: jeringa, trozo de algodón con alcohol o jabón y agua, y contenedor para objetos punzantes
- Compruebe la fecha de caducidad del medicamento. Si ha caducado, no lo utilice.
- Compruebe que la jeringa no está dañada y que la solución del medicamento sea transparente. Si no es así, utilice otra jeringa
- Asegúrese que conoce la cantidad que se va a inyectar
- Revise en la zona de su estómago si la última inyección le provocó enrojecimiento, cambio del color de la piel, hinchazón, supuración o dolor que aún persiste. Si esto ocurriera, consulte con su médico o enfermera
Instrucciones para inyectarse usted mismo Clexane:
(Instrucciones para jeringas sin dispositivo de seguridad)
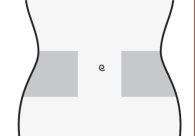
Preparación del lugar de inyección
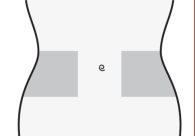
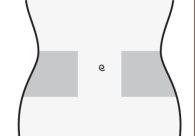
- Elija una zona en el lado derecho o izquierdo de su estómago. Al menos a 5 cm del ombligo y hacia cualquiera de los dos costados.
- No se inyecte dentro de los 5 cm que rodean su ombligo o alrededor del mismo si existen cicatrices o hematomas.
- Para inyectarse, alterne el lado izquierdo y derecho de su estómago, dependiendo de dónde se inyectó la última vez.
- Lávese las manos. Limpie (no frote) la zona en la que va a realizar la inyección con un trozo de algodón con alcohol o con jabón y agua.
- Siéntese o túmbese en una posición cómoda para que esté relajado. Compruebe que puede ver la zona en la que se va a inyectar. Lo más adecuado es en un diván, un sillón reclinable, o en una cama con cojines.
Selección de la dosis
- Retire cuidadosamente el capuchón de la aguja de la jeringa tirando de él. Deseche el capuchón.
- Antes de inyectarse no presione el émbolo para eliminar las burbujas de aire. Esto puede dar lugar a una pérdida de medicamento.
- Una vez que haya retirado el capuchón, no toque nada con la aguja. De este modo se asegurará que la aguja siga estando limpia (estéril).
- Cuando la cantidad de medicamento en la jeringa coincide con la dosis que le han prescrito, no hay necesidad de ajustar la dosis. Ahora ya está preparado para la administración de la inyección.
- Cuando la dosis depende de su peso corporal, podría necesitar ajustar la dosis en la jeringa para que coincida con la dosis prescrita. En este caso, podrá deshacerse del exceso de medicamento manteniendo la jeringa apuntando hacia abajo (para mantener la burbuja de aire en la jeringa) y expulsando el exceso en un contenedor.
- Podría aparecer una gota en el extremo de la aguja. Si esto sucede, hay que eliminar la gota antes de administrar la inyección dando golpecitos suaves a la jeringa con la aguja apuntando hacia abajo. Ahora ya está preparado para la administración de la inyección.
Administración de la inyección
- Sujete la jeringa con la mano que escribe (como si fuera un lápiz). Con la otra mano, pellizque suavemente, la zona que ha limpiado de su estómago, entre el dedo índice y el pulgar para formar un pliegue en la piel.
- Asegúrese de sostener este pliegue de piel mientras dure la inyección.
- Mantenga la jeringa de manera que la aguja apunte recta hacia abajo (verticalmente con un ángulo de 90º). Introduzca toda la aguja en el pliegue de piel.

- Presione el émbolo con su pulgar. De este modo administrará la medicación en el tejido graso del estómago. Complete la inyección usando todo el medicamento de la jeringa.
- Retire la aguja del lugar de inyección tirando recto de ella. Oriente la aguja alejada de si mismo y de otras personas. Ahora puede soltar el pliegue de piel.
Cuando haya finalizado
- Para evitar que le salga un hematoma, no frote la zona de inyección después de que se haya inyectado.
- Deposite la jeringa usada en el contenedor para objetos punzantes. Cierre bien la tapa del contenedor y coloque el contenedor fuera del alcance de los niños. Cuando el contenedor esté lleno, elimínelo tal como su médico o farmacéutico le haya indicado.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
Instrucciones para jeringas con dispositivo de seguridad automático tipo ERISTM:
Preparación del lugar de inyección
- Elija una zona en el lado derecho o izquierdo de su estómago. Al menos a 5 cm del ombligo y hacia cualquiera de los dos costados.
- No se inyecte dentro de los 5 cm que rodean su ombligo o alrededor del mismo si existen cicatrices o hematomas.
- Para inyectarse, alterne el lado izquierdo y derecho de su estómago, dependiendo de dónde se inyectó la última vez.
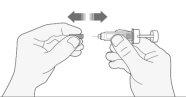
- Lávese las manos. Limpie (no frote) la zona en la que va a realizar la inyección con un trozo de algodón con alcohol o con jabón y agua.
- Siéntese o túmbese en una posición cómoda para que esté relajado. Compruebe que puede ver la zona en la que se va a inyectar. Lo más adecuado es en un diván, sillón reclinable, o en una cama con cojines.
Selección de la dosis
- Retire cuidadosamente el capuchón de la aguja de la jeringa tirando de él. Deseche el capuchón.
- Antes de inyectarse no presione el émbolo para eliminar las burbujas de aire. Esto puede dar lugar a una pérdida de medicamento.
- Una vez que haya retirado el capuchón, no toque nada con la aguja. De este modo se asegurará que la aguja siga estando limpia (estéril).
- Cuando la cantidad de medicamento en la jeringa coincide con la dosis que le han prescrito, no hay necesidad de ajustar la dosis. Ahora ya está preparado para la administración de la inyección.
- Cuando la dosis depende de su peso corporal, podría necesitar ajustar la dosis en la jeringa para que coincida con la dosis prescrita. En este caso, podrá deshacerse del exceso de medicamento manteniendo la jeringa apuntando hacia abajo (para mantener la burbuja de aire en la jeringa) y expulsando el exceso en un contenedor.
- Podría aparecer una gota en el extremo de la aguja. Si esto sucede, hay que eliminar la gota antes de administrar la inyección dando golpecitos suaves a la jeringa con la aguja apuntando hacia abajo. Ahora ya está preparado para la administración de la inyección.
Administración de la inyección
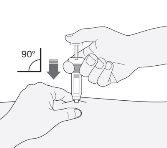
- Sujete la jeringa con la mano que escribe (como si fuera un lápiz). Con la otra mano, pellizque suavemente, la zona que ha limpiado de su estómago, entre el dedo índice y el pulgar para formar un pliegue en la piel.
- Asegúrese de sostener este pliegue de piel mientras dure la inyección.
- Mantenga la jeringa de manera que la aguja apunte recta hacia abajo (verticalmente con un ángulo de 90º). Introduzca toda la aguja en el pliegue de piel.
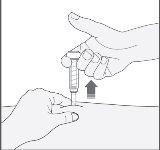
- Presione el émbolo con su pulgar. De este modo administrará la medicación en el tejido graso del estómago. Complete la inyección usando todo el medicamento de la jeringa.
- Retire la aguja del lugar de inyección tirando recto de ella. Una funda protectora cubrirá automáticamente la aguja. Ahora puede soltar el pliegue de piel. El dispositivo de seguridad sólo liberará la funda protectora cuando la jeringa se haya vaciado presionando profundamente el émbolo.
Cuando haya finalizado
- Para evitar que le salga un hematoma, no frote la zona de inyección después de que se haya inyectado.
- Deposite la jeringa usada en el contenedor para objetos punzantes. Cierre bien la tapa del contenedor y coloque el contenedor fuera del alcance de los niños. Cuando el contenedor esté lleno, elimínelo tal como su médico o farmacéutico le haya indicado.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
Instrucciones para jeringas con dispositivo de seguridad automático tipo PREVENTISTM:
Preparación del lugar de inyección
- Elija una zona en el lado derecho o izquierdo de su estómago. Al menos a 5 cm del ombligo y hacia cualquiera de los dos costados.
- No se inyecte dentro de los 5 cm que rodean su ombligo o alrededor del mismo si existen cicatrices o hematomas.
- Para inyectarse, alterne el lado izquierdo y derecho de su estómago, dependiendo de dónde se inyectó la última vez.
- Lávese las manos. Limpie (no frote) la zona en la que va a realizar la inyección con un trozo de algodón con alcohol o con jabón y agua.
- Siéntese o túmbese en una posición cómoda para que esté relajado. Compruebe que puede ver la zona en la que se va a inyectar. Lo más adecuado es en un diván, sillón reclinable, o en una cama con cojines.
Selección de la dosis
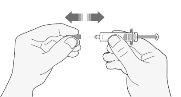
- Retire cuidadosamente el capuchón de la aguja de la jeringa tirando de él. Deseche el capuchón.
- Antes de inyectarse no presione el émbolo para eliminar las burbujas de aire. Esto puede dar lugar a una pérdida de medicamento.
- Una vez que haya retirado el capuchón, no toque nada con la aguja. De este modo se asegurará que la aguja siga estando limpia (estéril).
- Cuando la cantidad de medicamento en la jeringa coincide con la dosis que le han prescrito, no hay necesidad de ajustar la dosis. Ahora ya está preparado para la administración de la inyección.
- Cuando la dosis depende de su peso corporal, podría necesitar ajustar la dosis en la jeringa para que coincida con la dosis prescrita. En este caso, podrá deshacerse del exceso de medicamento manteniendo la jeringa apuntando hacia abajo (para mantener la burbuja de aire en la jeringa) y expulsando el exceso en un contenedor.
- Podría aparecer una gota en el extremo de la aguja. Si esto sucede, hay que eliminar la gota antes de administrar la inyección dando golpecitos suaves a la jeringa con la aguja apuntando hacia abajo. Ahora ya está preparado para la administración de la inyección.
Administración de la inyección
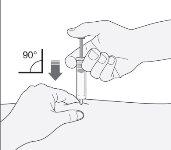
- Sujete la jeringa con la mano que escribe (como si fuera un lápiz). Con la otra mano, pellizque suavemente, la zona que ha limpiado de su estómago, entre el dedo índice y el pulgar para formar un pliegue en la piel.
- Asegúrese de sostener este pliegue de piel mientras dure la inyección.
- Mantenga la jeringa de manera que la aguja apunte recta hacia abajo (verticalmente con un ángulo de 90º). Introduzca toda la aguja en el pliegue de piel.
- Presione el émbolo con su pulgar. De este modo administrará la medicación en el tejido graso del estómago. Complete la inyección usando todo el medicamento de la jeringa.
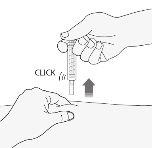
- Retire la aguja del lugar de inyección tirando recto de ella manteniendo el dedo en el émbolo. Oriente la aguja alejada de si mismo y de otras personas, y presione firmemente el émbolo para activar el sistema de seguridad. La funda protectora cubrirá automáticamente la aguja. Escuchará un “clic” que confirma la activación del sistema de seguridad. Ahora puede soltar el pliegue de piel.
Cuando haya finalizado
- Para evitar que le salga un hematoma, no frote la zona de inyección después de que se haya inyectado.
- Deposite la jeringa usada en el contenedor para objetos punzantes. Cierre bien la tapa del contenedor y coloque el contenedor fuera del alcance de los niños. Cuando el contenedor esté lleno, elimínelo tal como su médico o farmacéutico le haya indicado.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
Cambio de medicamento anticoagulante
- Cambio de Clexane a medicamentos para reducir la coagulación de la sangre denominados antagonistas de la vitamina K (como warfarina)
Su médico solicitará que le realicen la determinación en sangre de un parámetro denominado INR y le dirá cuándo debe interrumpir el tratamiento con Clexane.
- Cambio de medicamentos para reducir la coagulación de la sangre denominados antagonistas de la vitamina K (como warfarina) a Clexane
Interrumpa el uso del antagonista de la vitamina K. Su médico solicitará que le realicen la determinación en sangre de un parámetro denominado INR y le dirá cuándo empezar a utilizar Clexane.
- Cambio de Clexane a tratamiento con anticoagulantes orales directos
Interrumpa el uso de Clexane. Empiece a tomar el anticoagulante oral directo de 0 a 2 horas antes de cuando le hubiera tocado la siguiente inyección, y después continúe como habitualmente lo hace.
- Cambio de tratamiento con anticoagulante oral directo a Clexane
Deje de tomar el anticoagulante oral directo. No inicie el tratamiento con Clexane hasta pasadas 12 horas desde la última dosis del anticoagulante oral directo.
Si usa más Clexane del que debe
Si considera que ha usado demasiada cantidad o demasiado poco Clexane, informe inmediatamente a su médico, farmacéutico o enfermera, incluso si no presenta signos de que esté padeciendo algún problema. Si un niño se inyecta o traga Clexane accidentalmente, llévelo inmediatamente al servicio de urgencias de un hospital.
En caso de sobredosis o administración accidental, consulte inmediatamente a su médico o diríjase al servicio de urgencias del hospital más próximo llevando consigo este prospecto, o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada.
Si olvidó utilizar Clexane
Si olvidó administrarse una dosis, hágalo tan pronto como lo recuerde. No use una dosis doble en el mismo día para compensar las dosis olvidadas. Para asegurarse que no olvida ninguna dosis, puede serle de utilidad el uso de un diario.
Si interrumpe el tratamiento con Clexane
Es importante que usted siga recibiendo Clexane hasta que su médico decida interrumpir el tratamiento. Si deja de usarlo, se podría formar un coágulo de sangre, lo que puede ser muy peligroso.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico o enfermera.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Interrumpa el tratamiento con Clexanee informe inmediatamente a su médico o enfermerasi experimenta cualquier signo de reacción alérgica grave (como erupción, problemas para respirar o tragar, hinchazón de la cara, labios, lengua, cavidad bucal, garganta u ojos).
Interrumpa el tratamiento con enoxaparina e informe inmediatamente a su médico o enfermera si experimenta alguno de los siguientes síntomas:
- una erupción generalizada, roja y escamosa, con protuberancias bajo la piel y ampollas, acompañada de fiebre. Los síntomas suelen aparecer al inicio del tratamiento (pustulosis exantemática generalizada aguda).
Al igual que otros medicamentos similares para reducir los coágulos en sangre, Clexane podría causar sangrado. Esto podría poner en peligro su vida. En algunos casos el sangrado podría no ser evidente.
Informe inmediatamente a su médico si:
- tiene cualquier sangrado que no para por sí mismo
- tiene signos de sangrado excesivo (como sentirse muy débil, cansancio, palidez, o mareo con dolor de cabeza o hinchazón inexplicable).
Su médico podría decidir mantenerle bajo estricta observación o cambiar su medicación.
Debe informar inmediatamente a su médico:
- si presenta cualquier signo de bloqueo de un vaso sanguíneo por un coágulo de sangre como:
- dolor tipo calambre, enrojecimiento, calor, o hinchazón en una de sus piernas – que son síntomas de trombosis venosa profunda
- dificultad para respirar, dolor en el pecho, desmayo o tos con sangre – que son síntomas de embolismo pulmonar
- si tiene erupción cutánea dolorosa con puntos de color rojo oscuro bajo la piel que no desaparecen al presionarlos.
Su médico podría solicitar un análisis de sangre para comprobar el número de plaquetas.
Otros efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- sangrado
- aumento de las enzimas hepáticas.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- si aparecen hematomas con mayor frecuencia de lo habitual - esto podría deberse a un problema de la sangre debido a un número bajo de plaquetas
- placas rosadas en la piel - aparecen con mayor frecuencia en la zona en la que le han inyectado Clexane
- erupción en la piel (habones, urticaria)
- enrojecimiento y picor en la piel
- moratón o dolor en el lugar de inyección
- disminución del número de células rojas en sangre
- aumento del número de plaquetas en sangre
- dolor de cabeza.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- dolor de cabeza grave repentino - esto podría ser un signo de hemorragia en el cerebro
- sensación de sensibilidad a la palpación e hinchazón del estómago - podría ser indicativo de una hemorragia gástrica
- lesiones rojas y grandes en la piel, de forma irregular con o sin ampollas
- irritación en la piel (irritación local)
- amarilleamiento de la piel u ojos, y oscurecimiento del color de la orina - esto podría ser debido a un problema de hígado.
Raros(pueden afectar hasta 1 de cada 1.000 personas):
- reacción alérgica grave - los signos de esta reacción podrían incluir: erupción en la piel, problemas para tragar o respirar, hinchazón en los labios, cara, garganta o lengua
- aumento del potasio en sangre - esto es más probable que suceda en personas con problemas de riñón o diabetes. Su médico podrá comprobarlo realizando un análisis de sangre
- aumento del número de eosinófilos en sangre - su médico podrá comprobarlo realizando un análisis de sangre
- pérdida de pelo
- osteoporosis (una enfermedad en la que los huesos se pueden fracturar con mayor probabilidad)
- hormigueo, entumecimiento y debilidad en los músculos (especialmente en la parte inferior del cuerpo) cuando le han practicado una punción lumbar o una anestesia espinal
- pérdida de control de la vejiga o el intestino (de modo que no puede controlar sus necesidades)
- endurecimiento o nódulo en el lugar de inyección.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermera, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Clexane
No conservar a temperatura superior a 25°C. No congelar.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamentodespués de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa una fisura en la jeringa, partículas en suspensión en la disolución, o un color anómalo de la disolución (ver “Aspecto del producto y contenido del envase”).
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Clexane
- El principio activo es enoxaparina sódica
Cada ml contiene 150 mg de enoxaparina sódica, equivalente a 15.000 UI de actividad de anti-Xa
- Cada jeringa precargada con 0,8 ml contiene 12.000 UI (120 mg) de enoxaparina sódica
- El otro componente es agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Clexane es una solución inyectable en jeringa precargada de vidrio (con o sin dispositivo de seguridad automático), transparente, de incolora a amarillenta.
Se presenta en envases de de 2, 5, 6, 10, 20, 30, 50 jeringas precargadas, y en envases múltiples de 3 x 10 jeringas precargadas.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
sanofi-aventis, S.A.
C/ Roselló i Porcel, 21
08016 Barcelona
España
Responsable de la fabricación
Sanofi Winthrop Industrie
180 rue Jean Jaurès
94700 Maisons-Alfort
Francia
O
Sanofi-Aventis Private Co. Ltd
Budapest Logistics and Distribution Platform
Bdg. DC5, Campona utca1.
Budapest, 1225
Hungría
O
Sanofi-Aventis GmbH
Turm A, 29. OG, Wienerbergstraβe 11
1100 Viena
Austria
O
Sanofi Winthrop Industrie1051 Boulevard Industriel76580 Le TraitFrancia
O
Sanofi-Aventis Deutschland GmbH
Industriepark Höchst-Brüningstraβe 50
65926 Frankfurt am Main
Alemania
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Francia, Portugal: Lovenox.
Bélgica, Alemania, Grecia, Luxemburgo, Eslovenia, España: Clexane.
República Checa, Hungría, Irlanda, Polonia, Eslovaquia, Reino Unido: Clexane Forte.
Italia: Clexane T.
Finlandia, Noruega, Suecia: Klexane.
Fecha de la última revisión de este prospecto:febrero 2022
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia86.05 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CLEXANE 12.000 UI (120 mg)/ 0,8 ml SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 100 mg (10000 UI) enoxaparina sodica/mlPrincipio activo: enoxaparinFabricante: Sanofi Aventis S.A.Requiere recetaForma farmacéutica: INYECTABLE, 150 mg (15000 UI) /1 mlPrincipio activo: enoxaparinFabricante: Sanofi Aventis S.A.Requiere recetaForma farmacéutica: INYECTABLE, 20 mg (2000 UI) enoxaparina sodica/0,2 mlPrincipio activo: enoxaparinFabricante: Sanofi Aventis S.A.Requiere receta
Médicos online para CLEXANE 12.000 UI (120 mg)/ 0,8 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CLEXANE 12.000 UI (120 mg)/ 0,8 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















