
ANARTEX 300 MG/ML SOLUCION ORAL


Cómo usar ANARTEX 300 MG/ML SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Anartex®300 mg/ml solución oral
Oxibato de sodio
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Anartex y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Anartex
- Cómo tomar Anartex
- Posibles efectos adversos
- Conservación de Anartex
- Contenido del envase e información adicional
1. Qué es Anartex y para qué se utiliza
Anartex contiene el principio activo oxibato de sodio. Anartex actúa consolidando el sueño nocturno, aunque se desconoce su mecanismo de acción exacto.
Anartex se usa para tratar la narcolepsia con cataplejía en adultos, adolescentes y niños a partir de 7 años de edad.
La narcolepsia es un trastorno del sueño que puede incluir ataques de sueño durante las horas en que normalmente se está despierto, así como cataplejía, parálisis del sueño, alucinaciones e insomnio. La cataplejía es aparición repentina de debilidad o parálisis muscular sin pérdida de consciencia, en respuesta a una reacción emocional repentina como rabia, miedo, alegría, risa o sorpresa.
2. Qué necesita saber antes de empezar a tomar Anartex
No tomeAnartex
- si es alérgico al oxibato de sodio o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6);
- si tiene deficiencia de semialdehído-succínico-deshidrogenasa (un trastorno metabólico raro);
- si sufre una depresión grave;
- si está recibiendo tratamiento con medicamentos opioides o barbitúricos.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Anartex.
- si tiene problemas respiratorios o pulmonares (y especialmente si es obeso), ya que Anartex tiene el potencial para causar dificultad para respirar;
- si tiene o ha tenido depresión, pensamientos suicidas, ansiedad, psicosis (trastorno mental que puede implicar alucinaciones, habla incoherente o conducta desorganizada y agitada) o trastorno bipolar;
- si padece insuficiencia cardíaca, hipertensión (presión arterial elevada), problemas de hígado o riñón, es posible que le deban ajustar su dosis;
- si previamente ha consumido drogas o ha abusado de medicamentos;
- si sufre epilepsia, ya que no se recomienda el uso de Anartex en esta enfermedad;
- si padece porfiria (un trastorno metabólico raro).
Si padece alguno de estos problemas, informe a su médico antes de tomar Anartex.
Si mientras toma Anartex, sufre pérdidas de orina nocturnas e incontinencia (tanto urinaria como fecal), confusión, alucinaciones, episodios de sonambulismo o pensamiento anormal, deberá comunicárselo inmediatamente a su médico. Aunque estos efectos son poco frecuentes, si aparecen, son por lo general de naturaleza leve a moderada.
En las personas mayores, el médico seguirá cuidadosamente su evolución para comprobar si Anartex produce los efectos deseados.
Anartex tiene un potencial de abuso bien conocido. Se han dado casos de dependencia tras un uso ilícito del oxibato de sodio.
Su médico le preguntara si ha consumido cualquier droga antes de comenzar a tomar Anartex y mientras esté tomando este medicamento.
Niños y adolescentes
Anartex lo pueden tomar adolescentes y niños a partir de 7 años de edad cuando pesen más de 15 kg.
Anartex no pueden tomarlo niños menores de 7 años de edad o que pesen menos de 15 kg.
Si eres niño o adolescente, tu médico controlará tu peso corporal con regularidad.
Mientras el médico esté ajustando la dosis, lo que puede llevar una serie de semanas, los padres/cuidadores deben controlar cuidadosamente la respiración del niño durante las 2 primeras horas después de la ingesta del oxibato de sodio para evaluar si hay alguna anomalía en la respiración; por ejemplo, interrupción de la respiración durante periodos cortos mientras duerme, respiración ruidosa y un color azulado en los labios y la cara. Si se observan anomalías en la respiración, se debe buscar asistencia médica y el médico debe ser informado tan pronto como sea posible. Si se observa alguna anomalía después de la primera dosis, no debe administrarse la segunda dosis. Si no se observa ninguna anomalía, se puede administrar la segunda dosis. La segunda dosis no debe administrarse antes de 2,5 horas ni después de 4 horas tras la administración de la primera dosis.
Si ha tenido o está teniendo sensaciones molestas, especialmente si se siente muy triste o ha perdido el interés en la vida, es importante que informe a su médico o cuidador.
Uso deAnartexcon otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
En particular, Anartex no se debe usar junto con medicamentos que inducen el sueño y medicamentos que reducen la actividad del Sistema Nervioso Central (el Sistema Nervioso Central es la parte del cuerpo compuesto por el cerebro y la médula espinal):
También debe informar a su médico o farmacéutico si está usando alguno de los siguientes tipos de medicamentos:
- medicamentos que incrementan la actividad del sistema nervioso central y antidepresivos
- medicamentos que pueden metabolizarse de forma similar por el organismo (ej., valporato, fenitoína o etosuximida, que se utilizan para el tratamiento de las crisis epilépticas).
*topiramato (utilizado para el tratamiento de la epilepsia)
- si está tomando valproato, su dosis diaria de Anartex tendrá que ser ajustada (ver sección 3) ya que puede dar lugar a interacciones.
Toma deAnartexcon alcohol
No debe beber alcohol mientras esté tomando Anartex, ya que sus efectos pueden verse incrementados.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Ha habido muy pocas mujeres que hayan tomado oxibato durante su embarazo y algunas de ellas sufrieron abortos espontáneos. No se conoce el riesgo de tomar Anartex durante el embarazo, por lo que no se recomienda su uso en mujeres embarazadas o mujeres que estén tratando de quedarse embarazadas.
Las pacientes que tomen Anartex deben interrumpir la lactancia, ya que Anartex pasa a la leche materna. Se han observado cambios en el sueño en los lactantes de madres expuestas.
Conducción y uso de máquinas
Anartex puede afectarle si usted conduce o utiliza máquinas. No conduzca, no utilice maquinaria pesada, ni realice cualquier actividad que pueda ser peligrosa o que requiera alerta mental, durante al menos 6 horas después de la ingesta de Anartex. Cuando empiece a tomar Anartex por primera vez y hasta que sepa si le produce somnolencia al día siguiente, tenga especial cuidado cuando conduzca, opere con maquinaria pesada o haga cualquier otra actividad que pudiera resultar peligrosa o necesite un estado de alerta mental completo.
En pacientes pediátricos, se avisa a los médicos, padres o cuidadores que el tiempo de espera para realizar actividades que requieran un estado mental de alerta, coordinación motora o actividades que puedan tener un riesgo físico puede ser de más de 6 horas, dependiendo de la sensibilidad individual.
Anartexcontiene sodio
Este medicamento contiene 182,24 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada gramo. Esto equivale al 9,11 % de la ingesta diaria máxima de sodio recomendada para un adulto.
Consulte con su médico o farmacéutico si necesita 2 g de oxibato de sodio (Anartex) o más al día por un período prolongado, especialmente si le han recomendado una dieta baja en sal (sodio).
También es relevante para niños, donde la ingesta máxima diaria se considera proporcional a la de los adultos y se basa en las necesidades energéticas.
3. Cómo tomar Anartex
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Es importante que solo utilice la jeringa y los vasos dosificadores incluidos en la caja durante la preparación de las dosis de Anartex. La jeringa de Anartex tiene dos unidades de medición distintas: gramos (g) y mililitros (ml).
Adultos: toma de Anartex solo
- Para adultos, la dosis inicial recomendada es 4,5 g cada día, repartida en dos dosis separadas de 2,25 g.
- Su médico podrá aumentar gradualmente su dosis hasta un máximo de 9 g cada día repartidos en dos dosis separadas de 4,5 g.
- Tome Anartex vía oral dos veces cada noche:
- Tome la primera dosis al ir a acostarse y la segunda dosis de 2,5 a 4 horas más tarde. Puede necesitar un despertador para asegurarse que se despertará para tomar la segunda dosis.
- Los alimentos disminuyen la cantidad de Anartex que absorbe su organismo. Por lo tanto, es mejor tomar Anartex siempre a una hora determinada 2-3 horas después de las comidas.
- Prepare ambas dosis antes de acostarse.
- Tome las dosis dentro de las 24 horas posteriores a su preparación.
Adolescentes y niños a partir de 7 años que pesan 15 kg o más: toma de Anartex solo
Para los niños a partir de 7 años que pesen 15 kg o más, el médico calculará la dosis adecuada en función del peso corporal.
El médico calculará la dosis adecuada para ti. No superes la dosis que se te ha recetado.
Adultos: toma de Anartex con valproato
Si está tomando valproato junto con Anartex, su médico le ajustará la dosis de Anartex.
- Para adultos, la dosis inicial recomendada de Anartex cuando se utiliza junto con valproato es de 3,6 g cada día, dividida en dos dosis separadas de 1,8 g.
- Tome la primera dosis cuando se vaya a acostar y la segunda dosis de 2,5 a 4 horas después.
Adolescentes y niños a partir de 7 años que pesan 15 kg o más: toma de Anartex con valproato
Si estás tomando valproato junto con Anartex, tu médico te ajustará la dosis de Anartex.
Problemas hepáticos o renales
- Si tiene problemas de riñón, debe tener en cuenta las recomendaciones dietéticas para reducir la ingesta de sodio (sal).
- Si tiene problemas de hígado, la dosis inicial se debe reducir a la mitad. Su médico puede aumentar su dosis gradualmente.
Instrucciones para diluir Anartex
Existen otras soluciones orales de oxibato sódico, pero cada una de ellas tiene un modo de administración distinto. Lea con cuidado la forma de administración de este medicamento y si tiene dudas pregunte al farmacéutico.
Las siguientes instrucciones explican cómo preparar Anartex. Lea detenidamente las instrucciones y sígalas paso a paso.
Para ayudarle, el envase de Anartex contiene 1 frasco de medicamento, una jeringa graduada en gramos (g) y mililitros (ml), un adaptador y dos vasos dosificadores de aproximadamente 60 ml de capacidad con tapones de seguridad a prueba de niños.
Anartex contiene 300 mg de oxibato sódico por cada ml de solución. Una dosis de 4,5 g de oxibato sódico corresponde a 15 ml de solución. La jeringa que va a utilizar para administrar el medicamento y que se incluye en el envase está graduada en gramos (1,5g, 2,25g, 3,0g, 3,75g, 4,5g) y en mililitros (5 ml, 7,5 ml, 10 ml, 12,5 ml, 15 ml) habiendo marcas horizontales cada 0,25 gramos (o lo que es lo mismo, cada 1,25 ml). Los vasos dosificadores no contienen marcas de graduación. La siguiente tabla incluye las equivalencias de volumen de solución de Anartex y gramos de oxibato sódico que contiene. Usted deberá fijarse en la primera columna de la tabla (“Cantidad en gramos de Anartex”) al preparar su dosis con la jeringa graduada en gramos, ya que así es como se lo prescribirá su médico:
Cantidad en gramos de Anartex | Cantidad equivalente en ml de Anartex |
1,5 g | 5 ml |
2,25 g | 7,5 ml |
3,0 g | 10 ml |
3,75 g | 12,5 ml |
4,5 g | 15 ml |
Cada dosis medida de Anartex debe dispensarse en el vaso dosificador y diluirse en 60 ml de agua antes de la toma. Los 60 ml son apoximadamente el volumen del vaso dosificador que se incluye en el envase, que corresponde aproximadamente a 4 cucharadas soperas.
- Quite el tapón del frasco presionando hacia abajo, y desenrosque en sentido contrario a las agujas del reloj (hacia la izquierda). Después de quitar el tapón, ponga el frasco vertical sobre una mesa. Manteniendo el frasco en posición vertical, inserte en el cuello del frasco el adaptador a presión. Esto sólo debe hacerse la primera vez que se abra el frasco. El adaptador se puede dejar puesto en el frasco para los usos siguientes.
- A continuación, inserte la punta de la jeringa graduada en el centro de la apertura del frasco y haga presión firmemente (Ver Figura 1).
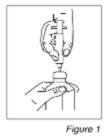
- Manteniendo el frasco y la jeringa en una mano, prepare la dosis prescrita con la otra mano tirando del émbolo. NOTA: El medicamento no fluirá en la jeringa a no ser que usted mantenga la botella en posición invertida (Ver Figura 2).
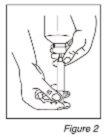
- Después de la preparación de la dosis, ponga el frasco en posición vertical y retire la jeringuilla del centro de la apertura del frasco. Vacíe el medicamento de la jeringa en uno de los vasos dosificadores proporcionados empujando el émbolo (Ver Figura 3). Repita este paso para el segundo vaso dosificador. Agregue entonces aproximadamente 60 ml de agua a cada vaso dosificador (60 ml son aproximadamente 4 cucharadas).
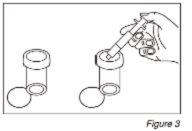
- Ponga los tapones de los vasos dosificadores y gire cada tapón en el sentido de las agujas del reloj (a la derecha) hasta sentir el click y ciérrelo en la posición a prueba de niños. (Ver Figura 4) Aclare la jeringa con agua.
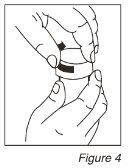
- Justo antes de ir a dormir:
- Los pacientes adultos deben colocar su segunda dosis cerca de su cama.
- Los padres o cuidadores de adolescentes y niños a partir de 7 años no deben dejar la segunda dosis cerca de la cama del niño o a su alcance.
- Puede necesitar un despertador para asegurarse que se levantará para tomar su segunda dosis, no antes de 2,5 horas y no más tarde de 4 horas después de su primera dosis.
A continuación
- Quite el tapón del primer vaso dosificador haciendo presión sobre el tapón de seguridad a prueba de niños y gírelo en sentido contrario a las agujas del reloj (hacia la izquierda).
- Beba la primera dosis sentado en la cama, tape el vaso, y luego acuéstese enseguida. En el caso de los niños que duermen más de 8 horas pero menos de 12, la primera dosis puede administrarse después de que el niño haya dormido de 1 a 2 horas.
- Cuando usted se despierte o despierte al niño entre 2,5 y 4 horas más tarde, quite el tapón del segundo vaso dosificador. Sentado en la cama, beba la segunda dosis justo antes de volver a acostarse para seguir durmiendo. Tape el segundo vaso.
Si considera que el efecto de Anartex es demasiado intenso o demasiado débil, comuníqueselo a su médico o farmacéutico.
Si toma másAnartexdel que debe
Los síntomas de sobredosis por Anartex pueden incluir agitación, confusión, movilidad alterada, dificultad respiratoria, visión borrosa, sudoración excesiva, dolor de cabeza, vómitos, conciencia disminuida que puede conducir a coma, crisis epiléptica, sed excesiva, calambres musculares y debilidad. Si usted toma más Anartex del que debe, o lo toma por accidente, solicite inmediatamente ayuda médica de urgencia. Debe llevar con usted la caja del medicamento, incluso si está vacío.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomarAnartex
Si olvidó tomar la primera dosis, tómela en cuanto lo recuerde y continúe con el procedimiento descrito previamente. Si omite la segunda dosis, salte esa dosis y no tome Anartex de nuevo hasta la próxima noche. No tome una dosis doble para compensar las dosis olvidadas.
Si no está seguro de si ha tomado Anartex
En caso de duda sobre la administración de una dosis, no vuelva a administrar la dosis para reducir el riesgo de sobredosis.
Si interrumpe el tratamiento conAnartex
Deberá seguir tomando Anartex mientras su médico se lo continúe prescribiendo. Si se interrumpe la medicación, los ataques de cataplejía pueden volver y puede experimentar insomnio, dolor de cabeza, ansiedad, vértigo, trastornos del sueño, somnolencia, alucinaciones y pensamiento anormal.
Si interrumpe el tratamiento con Anartex durante más de 14 días, debe consultar con su médico ya que debe volver a empezar el tratamiento con Anartex a partir de una dosis menor.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Éstos con frecuencia son de intensidad leve a moderada.
Adultos: efectos adversos más frecuentes observados en estudios clínicos(que se producen en el 10 % al 20 % de los pacientes):
- mareo
- náuseas
- dolor de cabeza
Si experimenta alguno de estos efectos adversos, informe a su médico inmediatamente.
Niños y adolescentes: efectos adversos más frecuentes observados en un estudio clínico:
- mojar la cama (18,3 %)
- náuseas (12,5 %)
- vómitos (8,7 %)
- pérdida de peso (8,7 %)
- disminución del apetito (6,7 %)
- dolor de cabeza (5,8 %)
? mareo (5,8 %)
- pensamientos suicidas (1 %)
- sentirse mentalmente mal (pérdida del contacto con la realidad) (1 %)
Si experimenta alguno de estos efectos adversos, informe a su médico inmediatamente.
Los efectos adversos en adultos y niños son los mismos. Si experimenta alguno de estos efectos adversos, informe a su médico inmediatamente:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- náuseas
- mareo
- dolor de cabeza
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- problemas para dormir como insomnio, sueños anómalos, parálisis del sueño, somnolencia, pesadillas, sonambulismo, mojar la cama, somnolencia diurna excesiva, dificultad para conciliar el sueño a mitad de noche
- sensación de borrachera, temblores, confusión o desorientación, visión borrosa, trastorno del equilibrio, caídas, sensación de “mareo” (vértigo)
- sentir los latidos del corazón, aumento de la presión arterial, falta de aliento
- vómitos, dolor de estómago, diarrea
- anorexia, disminución del apetito, pérdida de peso
- debilidad, cansancio, sedación
- sudoración
- depresión
- calambres musculares, hinchazón
- dolor en las articulaciones, dolor de espalda
- alteración de la atención, alteración de la sensibilidad esencialmente al tacto, sensación anómala del tacto, sabor anómalo
- ansiedad, nerviosismo
- incontinencia urinaria
- ronquidos, congestión nasal
- sarpullido
- inflamación de los senos, inflamación de nariz y garganta
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- psicosis (un trastorno mental que puede incluir alucinaciones, discurso incoherente o comportamiento desorganizado y agitado)
- paranoia, pensamiento anómalo, alucinaciones, agitación, intento de suicidio
- dificultad para conciliar el sueño, piernas inquietas
- falta de memoria
- mioclonía (contracciones involuntarias de los músculos)
- deposiciones involuntarias
- hipersensibilidad
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- convulsión
- disminución de la profundidad o la frecuencia de la respiración, cese corto de la respiración durante el sueño
- urticaria
- pensamientos suicidas, delirio, pensamientos de cometer actos violentos (incluido hacer daño a otras personas)
- irritabilidad, agresividad
- estado de ánimo eufórico
- ataque de pánico
- manía/trastorno bipolar
- sequedad de boca, deshidratación
- hinchazón de la cara (angioedema)
- bruxismo (bruxismo y mandíbula apretada)
- polaquiuria/urgencia miccional (mayor necesidad de orinar)
- acúfenos (ruido en los oídos, como por ejemplo, tintineo o zumbidos)
- trastorno de la alimentación relacionado con el sueño
- aumento del apetito
- pérdida de conciencia
- discinesia (p. ej., movimientos anómalos y descontrolados de las extremidades)
- caspa
- aumento del deseo sexual
- nocturia (orinar en exceso por la noche)
- sensación de ahogo
Si experimenta alguno de estos efectos secundarios, informe a su médico inmediatamente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Anartex
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el frasco después de (CAD). La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Tras la dilución en los vasos dosificadores, la preparación se debe utilizar dentro de las 24 horas posteriores.
Una vez abierto el frasco de Anartex, cualquier contenido que no se haya usado en 40 días tras su apertura deberá ser desechado.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo desahacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
Una vez finalizado deseche el envase, incluido las jeringas, el adaptador y los vasos dosificadores.
6. Contenido del envase e información adicional
Composición deAnartex
- El principio activo es oxibato de sodio. Cada ml contiene 300 mg de oxibato sódico.
- Los demás componentes son agua purificada, ácido málico e hidróxido de sodio.
Aspecto del producto y contenido del envase
Anartex se presenta en un frasco de plástico de color ámbar de 300 ml que contiene 300 ml de solución oral, cerrado con un tapón a prueba de niños. Cada envase contiene un frasco, un adaptador, una jeringa de plástico graduada en gramos (1,5g, 2,25g, 3.0g, 3,75g, 4,5g) y en mililitros (5 ml, 7,5 ml, 10 ml, 12,5 ml, 15 ml) habiendo marcas horizontales cada 0,25 gramos (o lo que es lo mismo, cada 1,25 ml). y dos vasos dosificadores con tapones a prueba de niños. Los vasos dosificadores no contienen marcas de graduación
Anartex es una solución clara, incolora y libre de partículas visibles.
Titular de la autorización de comercialización
Accord Healthcare S.L.U.
World Trade Center
Moll de Barcelona, s/n.
Edifici Est 6ª planta 08039 - Barcelona
España
Responsable de la fabricación
Medichem S.A.
Narcís Monturiol, 41 A
08970 Sant Joan Despí
Barcelona. España
o
Laboratorios Salvat, S.A.
Gall, 30-36
08950 Esplugues de Llobregat (Barcelona)
España
Fecha de la última revisión de este prospecto: Octubre 2021
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es/.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ANARTEX 300 MG/ML SOLUCION ORALForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 500 mg/mlPrincipio activo: Oxibato sodioFabricante: Laboratorios Normon S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 500 mg/mlPrincipio activo: Oxibato sodioFabricante: Zentiva K.S.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 500 mg/mlPrincipio activo: Oxibato sodioFabricante: Laboratorio Reig Jofre, S.A.Requiere receta
Médicos online para ANARTEX 300 MG/ML SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ANARTEX 300 MG/ML SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes













