
Rixubis 2000 UI/frasco pó e solvente para solução injetável

Pergunte a um médico sobre a prescrição de Rixubis 2000 UI/frasco pó e solvente para solução injetável

Como usar Rixubis 2000 UI/frasco pó e solvente para solução injetável
Introdução
Prospecto: informação para o utilizador
RIXUBIS250UI Pó edissolvente para solução injectável
RIXUBIS500UI Pó edissolvente para solução injectável
RIXUBIS1000UI Pó edissolvente para solução injectável
RIXUBIS2000UI Pó edissolvente para solução injectável
RIXUBIS3000UI Pó edissolvente para solução injectável
nonacog gama (factor IX humano de coagulação recombinante)
Leia todo o prospecto atentamente antes de começar ausar este medicamento porque contém informações importantes para si.
- Conservar este prospecto, pois pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é RIXUBIS e para que é utilizado
- O que precisa saber antes de começar a usar RIXUBIS
- Como usar RIXUBIS
- Posíveis efeitos adversos
- Conservação de RIXUBIS
- Conteúdo do envase e informação adicional
1. O que é RIXUBIS e para que é utilizado
RIXUBIS contém o princípio ativo nonacog gama e é um factor IX humano de coagulação. O Factor IX é um componente normal do sangue humano necessário para a sua correta coagulação, RIXUBIS é utilizado em pacientes com hemofilia B (doença de Christmas, um distúrbio hereditário do sangue causado por uma deficiência de factor IX). Actua substituindo o factor IX que falta para permitir que o sangue do paciente se coagule.
RIXUBIS é utilizado para o tratamento e a prevenção de hemorragias em pacientes com hemofilia B de todas as idades.
2. O que precisa saber antes de começar a usar RIXUBIS
Não use RIXUBIS
- se é alérgico a nonacog gama ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6)
- se é alérgico às proteínas de hámster
Advertências eprecauções
É possível que se produzam reações de hipersensibilidade de tipo alérgico com RIXUBIS. Detenha a perfusão e entre em contacto imediatamente com o seu médico ou procure atendimento médico urgente se experimentar os primeiros sinais das reações de hipersensibilidade/alergia como habão urticarial, erupção, tirantez no peito, sibilância, pressão arterial baixa ou anafilaxia (reação alérgica grave que pode causar dificuldade para engolir e/ou respirar, cara e/ou mãos vermelhas ou inchadas). É possível que o seu médico precise tratá-lo imediatamente em caso de estas reações. É possível que o seu médico também lhe realize um exame de sangue para comprovar se desenvolveu anticorpos neutralizantes da atividade (inibidores) frente ao medicamento, pois os inibidores podem desenvolver-se juntamente com as alergias. Os pacientes com inibidores de factor IX podem ter um maior risco de anafilaxia durante o tratamento posterior com factor IX.
Consulte imediatamente o seu médico se a hemorragia não se detém da maneira esperada ou se experimenta um aumento significativo do uso de RIXUBIS para controlar uma hemorragia. O seu médico realizará um exame de sangue para comprovar se desenvolveu anticorpos neutralizantes da atividade (inibidores) frente a RIXUBIS. O risco de desenvolver inibidores é maior em pacientes a quem não foi administrado anteriormente um medicamento substituto de factor IX ou nas primeiras fases do tratamento, ou seja, no caso de crianças pequenas.
A produção de factor IX no corpo é controlada pelo gene de factor IX. Os pacientes que têm mutações específicas do seu gene de factor IX, como por exemplo uma eliminação maior, talvez tenham mais probabilidades de ter inibidores de factor IX e uma reação alérgica nas primeiras fases com qualquer concentrado de factor IX. Por isso, se se sabe que si tem tal mutação, o seu médico o controlará com maior cuidado para detectar sinais de uma reação alérgica.
Se padece doença hepática ou cardíaca, ou se se submeteu recentemente a uma intervenção cirúrgica importante, informe o seu médico, pois existe um maior risco de complicações na coagulação do sangue.
Foram notificados casos de distúrbios renais (síndrome nefrótico) após a administração de doses elevadas de factor IX em pacientes com hemofilia B que tinham inibidores de factor IX e antecedentes de reações alérgicas.
Sempre que seja possível, anote o nome do medicamento e o número do lote cada vez que utilize RIXUBIS (por exemplo, no seu diário) para manter um registo dos medicamentos e dos lotes que utilizou.
Uso de RIXUBIS com outros medicamentos
Informe o seu médico se está a utilizar, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento. Não foram notificadas interações de RIXUBIS com outros medicamentos.
Gravidez, lactação efertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento. A hemofilia B aparece em muito raras ocasiões em mulheres.
Condução euso de máquinas
A influência de RIXUBIS sobre a capacidade para conduzir e utilizar máquinas é nula.
RIXUBIS contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por frasco, ou seja, praticamente não tem sódio. No entanto, dependendo do seu peso corporal e da sua dose de RIXUBIS, pode receber mais de um frasco. Isto deve ser tido em conta se segue uma dieta pobre em sódio.
3. Como usar RIXUBIS
O tratamento com RIXUBIS será iniciado por um médico com experiência no tratamento de pacientes com hemofilia B.
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico.
O seu médico decidirá a dose de RIXUBIS que lhe será administrada. Esta dose e a duração dependerão da gravidade da sua deficiência de factor IX, da localização e da extensão da hemorragia, bem como do seu estado clínico, da idade e da rapidez com que o seu corpo consome o factor IX, que deve ser verificada regularmente.
O seu médico ou enfermeiro lhe administrarão RIXUBIS mediante perfusão intravenosa (IV) após a reconstituição do pó com o dissolvente fornecido. Si ou qualquer outra pessoa também podem administrar a injeção de RIXUBIS, mas apenas após receberem a formação adequada.
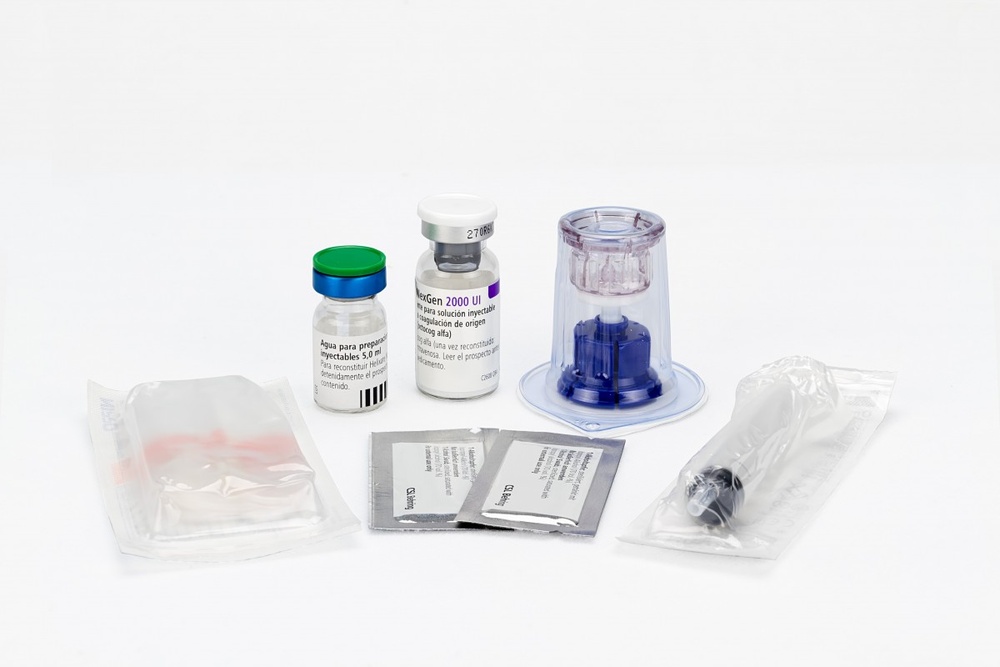
Reconstituição e administração
- Para a reconstituição, utilize apenas o dissolvente e o dispositivo de reconstituição (BAXJECT II) incluídos no envase.
- Para a administração é necessário o uso de uma seringa luer lock.
- Não utilize se o equipamento BAXJECT II, o sistema estéril de proteção ou o seu envase estiver danificado ou mostrar algum sinal de deterioração.
Reconstituição
Utilizar técnica asséptica:
- Se o medicamento se encontrar na geladeira, retire da geladeira os frascos de pó e de dissolvente de RIXUBIS e espere até que atinjam a temperatura ambiente (entre 15°C e 30°C).
- Lave as mãos com sabão e água morna.
- Retire os protetores dos frascos de pó e dissolvente.
- Limpe os tampões com as toalhetas impregnadas de álcool. Coloque os frascos em uma superfície plana e limpa.
- Abrir o invólucro do equipamento BaxJect II retirando a tampa de papel sem tocar o interior (Fig. a). Não retire o equipamento do invólucro.
- Dê a volta ao invólucro e insira a ponta de plástico através do tampão do dissolvente. Pegue o invólucro pelo seu extremo e retire o equipamento BaxJect II do seu invólucro (Fig. b). Não retire o protetor azul do equipamento BAXJECT II.
- Com Baxject II unido ao frasco de dissolvente, inverta o sistema de tal forma que o frasco de dissolvente esteja na parte superior do equipamento. Insira a ponta de plástico branca dentro do tampão do frasco de pó RIXUBIS. O vácuo fará com que o dissolvente penetre no frasco de pó RIXUBIS (Fig. c).
- Agite suavemente até que todo o material se tenha dissolvido. O medicamento se dissolve rapidamente (em cerca de 2 minutos). Certifique-se de que RIXUBIS esteja completamente dissolvido, se não for assim, toda a solução reconstituída não passará através do filtro do equipamento. Devem ser inspecionados visualmente os medicamentos reconstituídos para detectar partículas ou decoloração antes da administração. A solução deve ser transparente ou ligeiramente opalescente. Não utilize soluções turvas ou com depósitos.
Fig. aFig. bFig. c



Não refrigere a preparação após a reconstituição.
Utilize imediatamente.
Administração
Utilizar técnica asséptica:
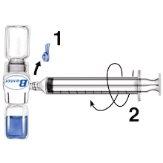
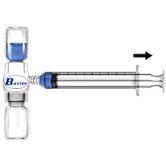
- Retire o protetor azul do equipamento BAXJECT II. Não introduza ar na seringa. Conecte a seringa ao equipamento BAXJECT II (Fig. d).
- Inverta o sistema (o frasco com a solução reconstituída na parte superior). Introduza a solução reconstituída na seringa, puxando o êmbolo para trás lentamente (Fig. e).
- Desconecte a seringa.
- Conecte uma agulha de perfusão com aletas à seringa. Injete por via intravenosa. A solução deve ser administrada lentamente, a uma velocidade determinada de acordo com o nível de confort do paciente, que não exceda 10 ml por minuto.
Fig. dFig. e
Sempre que seja possível, anote o nome do medicamento e o número do lote cada vez que utilize RIXUBIS (por exemplo, no seu diário) para manter um registo dos medicamentos e dos lotes que utilizou.
A eliminação do medicamento não utilizado e de todos os materiais que estiveram em contacto com ele será realizada de acordo com a regulamentação local.
Se usar mais RIXUBIS do que deve
Siga exactamente as instruções de administração de RIXUBIS indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico. Se for injetada uma dose maior de RIXUBIS do que a recomendada, consulte o seu médico o mais breve possível.
Se esquecer de usar RIXUBIS
Não se injete uma dose dupla para compensar as doses esquecidas. Administre a próxima injeção como está estabelecido e continue como o seu médico indicou.
Se interromper o tratamento com RIXUBIS
Não deixe de usar RIXUBIS sem consultar o seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
É possível que se produzam reações de hipersensibilidade de tipo alérgico com RIXUBIS. Entre estas reações incluem-se sensações de ardor e picadas no local de perfusão, calafrios, rubor, letargia, inquietude, formigamento, habão urticarial, picor e sarpullido, pressão arterial baixa, frequência cardíaca rápida, tirantez no peito, sibilância, inchaço da garganta, anafilaxia (reação alérgica grave), dor de cabeça, náuseas e vómitos. Consulte imediatamente o seu médico se experimentar estes sinais. É possível que o seu médico precise tratá-lo imediatamente em caso de estas reações (ver secção 2 ‘Advertências e precauções’).
Foram observados os seguintes efeitos adversos com RIXUBIS:
Efeitos adversos frequentes(podem afetar até 1 em cada 10 pacientes)
- alteração do gosto
- dor nas extremidades.
Efeitos adversos com frequência desconhecida(não se pode estimar a frequência a partir dos dados disponíveis)
- reações alérgicas (hipersensibilidade).
Não foram observados com este medicamento problemas causados por uma coagulação excessiva do sangue (episódios tromboembólicos), mas podem ocorrer com qualquer produto de factor IX. Entre estes, incluem-se ataques cardíacos, coágulos de sangue nas veias ou no pulmão.
Comunicaçãode efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de RIXUBIS
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no embalagem exterior e na etiqueta do frasco após CAD. A data de validade é o último dia do mês que se indica.
Conservar abaixo de 30°C.
Não congelar.
Utilize a solução reconstituída imediatamente.
Não utilize RIXUBIS se a solução não for incolor e transparente.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informação adicional
Composição de RIXUBIS
- O princípio ativo é nonacog gama (fator IX humano de coagulação recombinante). Cada frasco contém nominalmente 250, 500, 1000, 2000 ou 3000 UI, correspondentes a uma concentração de 50, 100, 200, 400 ou 600 UI/ml após a reconstituição com 5 ml de solvente.
- Os demais componentes do pó são sacarose, manitol, cloreto de sódio, cloreto de cálcio, L-histidina, polissorbato 80.
Frasco de solvente: 5 ml de água esterilizada para preparações injetáveis.
Aspecto do produto econteúdo do frasco
RIXUBIS é fornecido como pó e solvente para solução injetável.
O conteúdo do frasco é o seguinte:
- um frasco de pó RIXUBIS 250, 500, 1000, 2000 ou 3000 UI em um frasco de vidro com um fecho de borracha
- um frasco de 5 ml de água esterilizada para preparações injetáveis em um frasco de vidro com um fecho de borracha
- um BAXJECT II (equipamento de reconstituição sem agulhas)
Título da autorização de comercialização
Baxalta Innovations GmbH
Industriestrasse 67
A-1221 Viena
Tel.: 800 66838470 E-mail: [email protected]
Responsável pela fabricação
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B-7860 Lessines
Bélgica
Data da última revisão deste prospecto 01/2020
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑
Esta informação está destinada apenas a profissionais do setor sanitário:
Supervisão do tratamento
Durante o tratamento, é recomendada a determinação adequada dos níveis de fator IX para calcular a dose que deve ser administrada e a frequência das perfusões repetidas. Os pacientes individuais podem diferir em sua resposta ao fator IX com diferentes meias-vidas e recuperações. A dose baseada no peso corporal pode requerer um ajuste em pacientes com baixo peso ou sobrepeso. No caso particular de intervenções cirúrgicas importantes, é indispensável uma supervisão precisa da terapia de substituição mediante análise da coagulação (atividade do fator IX de plasma).
Para garantir que se alcançou o nível plasmático de atividade de fator IX desejado, é aconselhável realizar um controle exhaustivo utilizando um ensaio adequado de atividade de fator IX e, se necessário, devem ser aplicados os ajustes adequados à dose e à frequência das perfusões repetidas. Ao utilizar o ensaio in vitro de coagulação em uma etapa baseado no tempo de tromboplastina (aPTT) para determinar a atividade do fator IX em amostras sanguíneas de pacientes, os resultados de atividade do fator IX podem ser significativamente afetados pelo tipo de reagente de aPTT e pelo padrão de referência utilizado no ensaio. Isso é especialmente importante ao mudar o laboratório e/ou os reagentes utilizados no ensaio.
Posologia
A dose e a duração da terapia de substituição dependem da gravidade da deficiência de fator IX, da localização e da extensão da hemorragia, bem como do estado clínico, da idade e dos parâmetros farmacocinéticos de fator IX do paciente, como a recuperação incremental e meia-vida.
O número de unidades de fator IX administradas é expresso em unidades internacionais (UI), que estão relacionadas com o padrão atual da OMS para produtos de fator IX. A atividade de fator IX no plasma é expressa como um percentual (relativo ao plasma humano normal) ou em unidades internacionais (relativas a um padrão internacional para o fator IX no plasma).
Uma unidade internacional de atividade de fator IX é equivalente à quantidade de fator IX existente em um ml de plasma humano normal.
População adulta
Tratamento a demanda O cálculo da dose necessária de fator IX baseia-se no achado empírico de que 1 unidade internacional de fator IX por kg de peso corporal incrementa a atividade de fator IX do plasma em 0,9 UI/dl (intervalo de 0,5 a 1,4 UI/dl) ou 0,9% de atividade normal em pacientes de 12 anos de idade ou mais (para obter informações adicionais, ver seção 5.2).
A dose necessária é determinada utilizando a seguinte fórmula:
Unidades requeridas | = | peso corporal (kg) | x | aumento desejado de fator IX (%) ou (UI/dl) | x | recíproco de recuperação observada (dl/kg) |
Para uma recuperação incremental de 0,9 UI/dl por UI/kg, a dose é calculada da seguinte maneira:
Unidades requeridas | = | peso corporal (kg) | x | aumento desejado de fator IX (%) ou (UI/dl) | x | 1,1 dl/kg |
A quantidade que deve ser administrada e a frequência da administração devem estar sempre orientadas para a eficácia clínica no caso concreto.
No caso dos episódios hemorrágicos seguintes, a atividade de fator IX não deve ser inferior ao nível de atividade plasmática dada (em % do normal ou UI/dl) no período correspondente. Pode-se utilizar a seguinte tabela como guia de dosificação em episódios hemorrágicos e cirurgia:
Grau de hemorragia / tipo de procedimento cirúrgico | Nível de fator IX requerido (%) ou(UI/dl) | Frequência de dose (horas) / duração da terapia (dias) |
Hemorragia Hemartrose incipiente ou hemorragia muscular ou oral Hemartrose mais extensa, hemorragia muscular ou hematoma Hemorragia com risco vital. | 20 – 40 30 – 60 60 – 100 | Repetir cada 24 horas. Pelo menos 1 dia, até que o episódio hemorrágico, segundo indique a dor, se resolva ou se logre a curação. Repetir perfusão cada 24 horas durante 3 – 4 dias ou mais, até que cesse a dor e a incapacidade aguda. Repetir perfusão cada 8 a 24 horas até superar o perigo. |
Cirurgia Cirurgia menor, incluindo extração dental | 30 – 60 | Cada 24 horas, pelo menos 1 dia, até lograr a curação. |
Operação importante | 80 – 100 (pré e pós-operatório) | Repetir perfusão cada 8 a 24 horas até que se consiga uma curação adequada da ferida, e luego pelo menos outros 7 dias de terapia para manter uma atividade de fator IX de 30% a 60% (UI/dl). |
É especialmente importante uma supervisão cuidadosa da terapia de substituição nos casos de operação importante ou hemorragia potencialmente mortal.
Profilaxia
Para a profilaxia de longa duração contra hemorragias em pacientes com hemofilia B grave, as doses normais são de 40 a 60 UI de fator IX por quilograma de peso corporal a intervalos de 3 a 4 dias para os pacientes de 12 anos de idade ou mais. Em alguns casos, dependendo dos parâmetros farmacocinéticos, da idade, do fenótipo de hemorragia e da atividade física do paciente, pode ser necessário intervalos de dosificação mais curtos ou doses mais altas.
Perfusão contínua
Não administre RIXUBIS por perfusão contínua.
População pediátrica
Pacientes de 12 a 17 anos de idade:
A posologia é a mesma para os adultos e os pacientes pediátricos de 12 a 17 anos.
Pacientes menores de 12 anos:
Tratamento a demanda:
O cálculo da dose de fator IX requerida baseia-se no achado empírico de que 1 unidade internacional de fator IX por kg de peso corporal incrementa a atividade de fator IX do plasma em 0,7 UI/dl (intervalo de 0,31 a 1,0 UI/dl) ou 0,7% da atividade normal em pacientes menores de 12 anos de idade (para obter informações adicionais, ver seção 5.2).
A dosagem requerida é determinada mediante a seguinte fórmula:
Pacientes menores de 12 anos de idade:
Unidades requeridas | = | peso corporal (kg) | x | aumento desejado de fator IX (%) ou (UI/dl) | x | recíproco de recuperação observada (dl/kg) |
Para uma recuperação incremental de 0,7 UI/dl por UI/kg, a dose é calculada da seguinte maneira:
Unidades requeridas | = | peso corporal (kg) | x | aumento desejado de fator IX (%) ou (UI/dl) | x | 1,4 dl/kg |
Pode-se utilizar a mesma tabela para os adultos como guia de dosificação em episódios hemorrágicos e cirurgia (ver anterior).
Profilaxia:
O intervalo de doses recomendadas para pacientes pediátricos menores de 12 anos de idade é de 40 a 80 UI/kg a intervalos de 3 a 4 dias. Em alguns casos, dependendo dos parâmetros farmacocinéticos, da idade, do fenótipo de hemorragia e da atividade física do paciente, pode ser necessário intervalos de dosificação mais curtos ou doses mais altas.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a Rixubis 2000 UI/frasco pó e solvente para solução injetávelForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requer receita médicaForma farmacêutica: INJETÁVEL, 2.000 UISubstância ativa: coagulation factor IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requer receita médicaForma farmacêutica: INJETÁVEL, 250 UISubstância ativa: coagulation factor IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requer receita médica
Alternativas a Rixubis 2000 UI/frasco pó e solvente para solução injetável noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a Rixubis 2000 UI/frasco pó e solvente para solução injetável em Polónia
Alternativa a Rixubis 2000 UI/frasco pó e solvente para solução injetável em Ukraine
Médicos online para Rixubis 2000 UI/frasco pó e solvente para solução injetável
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de Rixubis 2000 UI/frasco pó e solvente para solução injetável – sujeita a avaliação médica e regras locais.














