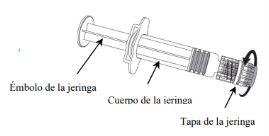
NIMENRIX pó e solvente para solução injetável em seringa pré-carregada

Pergunte a um médico sobre a prescrição de NIMENRIX pó e solvente para solução injetável em seringa pré-carregada

Como usar NIMENRIX pó e solvente para solução injetável em seringa pré-carregada
Introdução
Bula:informação para o utilizador
Nimenrix pó e diluentepara solução injectável em seringa pré-cheia
Vacina conjugada contra meningococo dos grupos A, C, W-135 e Y
Leia todo o folheto informativo atentamente antes de receber esta vacina, porque contém informações importantes para si.
- Conserva este folheto informativo, porque pode ter que o ler novamente.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi prescrito apenas para si ou para o seu filho e não deve ser dado a outras pessoas.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste folheto informativo. Ver secção 4.
Este folheto informativo foi escrito assumindo que a pessoa que vai receber a vacina é a que o vai ler. No entanto, a vacina pode ser administrada a adultos e crianças, de modo que é possível que o leia por seu filho.
Conteúdo do folheto informativo
- O que é Nimenrix e para que é utilizado
- O que precisa saber antes de receber Nimenrix
- Como é administrado Nimenrix
- Posíveis efeitos adversos
- Conservação de Nimenrix
- Conteúdo do envase e informações adicionais
1. O que é Nimenrix e para que é utilizado
O que é Nimenrix e para que é utilizado
Nimenrix é uma vacina que ajuda a proteger contra as infecções causadas pela bactéria (germe) chamada “Neisseria meningitidis”dos tipos A, C, W-135 e Y.
“Neisseria meningitidis”dos tipos A, C, W-135 e Y pode produzir doenças graves, tais como:
- meningite – uma infecção do tecido que reveste o cérebro e a medula espinhal.
- septicemia – uma infecção do sangue.
Estas infecções são transmitidas facilmente de uma pessoa para outra e, se não forem tratadas, podem causar a morte.
Nimenrix pode ser administrado a adultos, adolescentes, crianças e lactentes a partir das 6 semanas de idade.
Como funciona Nimenrix
Nimenrix ajuda o seu organismo a produzir a sua própria proteção (anticorpos) contra as bactérias. Estes anticorpos ajudam a proteger contra as doenças.
Nimenrix apenas o protegerá contra as infecções causadas pela bactéria “Neisseria meningitidis”dos tipos A, C, W-135 e Y.
2. O que precisa saber antes de receber Nimenrix
Não devem administrar-lhe Nimenrix se:
- é alérgico aos princípios ativos ou a algum dos outros componentes desta vacina (incluídos na secção 6).
Os sinais de uma reação alérgica podem incluir erupção cutânea com picazón, dificuldade para respirar e inchação do rosto ou da língua. Procure o seu médico imediatamente se experimentar algum destes sinais.
Se não tiver certeza, fale com o seu médico ou enfermeiro antes de receber Nimenrix.
Advertências e precauções
Consulte o seu médico ou enfermeiro antes de receber esta vacina se:
- tiver uma infecção com febre elevada (acima de 38 °C). Se este for o caso, não devem administrar-lhe a vacina até que se sinta melhor. Uma infecção de pouca importância, como um resfriado, não deveria ser um problema. No entanto, consulte antes com o seu médico ou enfermeiro.
- tiver um problema de coagulação ou lhe apareçam cardenais com facilidade.
Se se encontrar em alguma das circunstâncias acima (ou não tiver certeza), consulte com o seu médico ou enfermeiro antes de receber Nimenrix.
Pode ser que Nimenrix não proteja completamente todos os vacinados. Se tiver um sistema imunológico débil (por exemplo, devido a uma infecção por VIH ou a medicamentos que afetam o sistema imunológico) é possível que não se beneficie ao máximo da vacinação com Nimenrix.
Antes ou depois de qualquer injeção, pode ocorrer um desmaio (especialmente em adolescentes), por isso deve informar o seu médico ou enfermeiro se si ou o seu filho se desmaiou em ocasiões anteriores após a administração de uma injeção.
Outros medicamentos e Nimenrix
Informa o seu médico ou enfermeiro se está a utilizar ou utilizou recentemente qualquer outro medicamento, incluindo outras vacinas e medicamentos adquiridos sem receita.
Pode ser que Nimenrix não seja tão eficaz se está a utilizar medicamentos que afetam o seu sistema imunológico.
Em lactentes, Nimenrix pode ser administrado simultaneamente com vacinas combinadas difteria – tétano – tosferina acelular (DTPa), incluindo tosferina acelular (DTPa) com hepatite B, poliovírus inativado ou Haemophilus influenzaetipo b (VHB, IPV ou Hib), como a vacina DTPa-VHB-IPV/Hib e a vacina conjugada antineumocócica 10-valente.
Desde 1 ano de idade e maiores, Nimenrix pode ser administrado ao mesmo tempo que com alguma das seguintes vacinas: hepatite A (VHA) e hepatite B (VHB), a vacina do sarampo-papeira-rubéola (SRP, tríplice viral), a vacina do sarampo-papeira-rubéola-varicela (SRPV), a vacina conjugada antineumocócica 10-valente ou a vacina antigripal sazonal não adjuvada.
No segundo ano de vida, Nimenrix também pode ser administrado ao mesmo tempo com difteria – tétano – tosferina acelular (DTPa), incluindo tosferina acelular (DTPa) com hepatite B, poliovírus inativado ou Haemophilus influenzaetipo b (VHB, IPV ou Hib), como a vacina DTPa-VHB-IPV/Hib e a vacina conjugada antineumocócica 13-valente.
Em pessoas entre 9 e 25 anos, Nimenrix pode ser administrado ao mesmo tempo que a vacina do vírus do papiloma humano [tipos 16 e 18] e uma vacina combinada de difteria (conteúdo de antígeno reduzido), tétano e tosferina acelular.
Seempre que seja possível, a administração de Nimenrix e uma vacina que contenha toxoide tetânico, como a vacina DTPa-VHB-IPV/Hib, será realizada ao mesmo tempo ou Nimenrix será administrado pelo menos um mês antes da vacina que contenha toxoide tetânico.
Cada vacina será administrada em locais de injeção diferentes.
Gravidez e lactação
Se está grávida, acredita que possa estar grávida, tem intenção de engravidar, ou está em período de amamentação, consulte o seu médico antes de receber Nimenrix.
Condução e uso de máquinas
Não é provável que Nimenrix afete a sua capacidade para conduzir ou usar máquinas. No entanto, não conduza ou use máquinas se não se sentir bem.
Nimenrix contém sódio
Este medicamento contém menos de 23 mg (1 mmol) de sódio por dose: isto é, é essencialmente “isento de sódio”.
3. Como é administrado Nimenrix
O seu médico ou enfermeiro administrará Nimenrix.
Nimenrix sempre é injectado num músculo, normalmente na parte superior do braço ou coxa.
Primovacinação
Lactentes de 6 semanas a menos de 6 meses de idade
Duas injeções administradas com 2 meses de diferença, por exemplo, aos 2 e 4 meses de idade (a primeira injeção pode ser administrada a partir das 6 semanas de idade).
Lactentes de 6 meses de idade, crianças, adolescentes e maiores
Uma injeção.
Dose de reforço
Lactentes de 6 semanas a menos de 12 meses de idade:
Uma dose de reforço aos 12 meses de idade, pelo menos 2 meses após a última dose de Nimenrix.
Pessoas previamente vacinadas de 12 meses de idade e maiores:
Informa o seu médico se lhe foi administrada anteriormente uma injeção de outra vacina antimeningocócica diferente de Nimenrix.
O seu médico indicará se precisa de uma injeção adicional de Nimenrix e quando a precisa, especialmente se si ou o seu filho:
- recebeu a primeira dose aos 6-14 meses de idade e poderia ter um risco aumentado de infecção causada por Neisseria meningitidisdos tipos W-135 ou Y
- recebeu a dose há mais de um ano aproximadamente e poderia ter risco de infecção causada por Neisseria meningitidisdo tipo A
- recebeu a primeira dose aos 12-23 meses de idade e poderia ter um risco aumentado de infecção causada por Neisseria meningitidisdos tipos A, C, W-135 ou Y
Será informado quando deve regressar si ou o seu filho para que lhe sejam administradas as próximas injeções. Se si ou o seu filho não receber uma das injeções programadas, é importante que solicite outra consulta.
Certifique-se de que si ou o seu filho completa o ciclo completo de vacinação.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram. Com este medicamento podem ocorrer os seguintes efeitos adversos:
Muito frequentes (podem ocorrer com mais de 1 de cada 10 doses da vacina):
- febre
- fadiga
- dor de cabeça
- sensação de adormecimento
- perda de apetite
- sensação de irritabilidade
- inchação, dor e vermelhidão no local onde foi administrada a injeção.
Frequentes (podem ocorrer até com 1 de cada 10 doses da vacina):
- cardenais (hematomas) no local onde foi administrada a injeção
- problemas de estômago e de digestão, tais como diarreia, vómitos e náuseas.
- erupção (lactentes)
Pouco frequentes (podem ocorrer até com 1 de cada 100 doses da vacina):
- erupção
- habões
- picazón
- choro não habitual
- sensação de tontura
- músculos doloridos
- dor nos braços ou nas pernas
- malestar geral
- dificuldade para dormir
- sensibilidade diminuída, especialmente na pele
- reações no local onde foi administrada a injeção, tais como picazón, sensação de calor ou entorpecimento ou aparecimento de um caroço duro
- reação alérgica
Raros (podem ocorrer até com 1 de cada 1.000 doses da vacina):
- ataques (convulsões) relacionados com uma febre elevada
Frequência não conhecida (não pode ser estimada a partir dos dados disponíveis):
- inchação no local da injeção e vermelhidão; isto pode afetar uma área extensa do membro onde é administrada a vacina
- gânglio linfático inflamado
- reação alérgica grave
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste folheto informativo. Também pode comunicá-los directamente através do Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Nimenrix
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece no envase. A data de validade é o último dia do mês que se indica.
- Conservar em frigorífico (entre 2 °C e 8 °C).
- Conservar no embalagem original para protegê-lo da luz.
- Não congelar.
- Os medicamentos não devem ser deitados fora pela sanita nem para o lixo. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Nimenrix
Após a reconstituição, 1 dose (0,5 ml) contém: Polissacarídeo de Neisseria meningitidisdo grupo A Polissacarídeo de Neisseria meningitidisdo grupo C Polissacarídeo de Neisseria meningitidisdo grupo Y Conjugado com toxoide tetânico como proteína transportadora | 5 microgramas 5 microgramas 5 microgramas 5 microgramas 44 microgramas |
- Os outros componentes são:
- No pó: sacarose e trometamol
- No diluente: cloreto de sódio (ver seção 2 “Nimenrix contém sódio”) e água para preparações injetáveis
Aspecto do produto e conteúdo do envase
Nimenrix é um pó e diluente para solução injetável.
Nimenrix é fornecido como um pó ou pasta de cor branca em um frasco de vidro de dose única e um diluente transparente e incolor em uma seringa pré-carregada.
Ambos devem ser misturados antes do uso. A aparência da vacina misturada será uma solução transparente e incolor.
Nimenrix está disponível em embalagens de 1 ou 10 com ou sem agulhas.
Pode ser que apenas alguns tamanhos de embalagens sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização: Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Bruxelas Bélgica | Fabricante responsável pela liberação dos lotes: Pfizer Manufacturing Belgium N.V. Rijksweg 12 2870 Puurs-Sint-Amands Bélgica |
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica Luxemburgo Pfizer S.A./N.V. Tel: + 32 (0)2 554 62 11 | Lituânia Pfizer Luxembourg SARL filial em Lituânia Tel: + 370 52 51 4000 |
| Hungria Pfizer Kft Tel: +36 1 488 3700 |
República Tcheca Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: + 35621 344610 |
Dinamarca Pfizer ApS Tlf.: + 45 44 201 100 | Países Baixos Pfizer BV Tel: +31 (0)800 63 34 636 |
Alemanha Pfizer Pharma GmbH Tel: + 49 (0)30 550055-51000 | Noruega Pfizer AS Tlf: +47 67 526 100 |
Estônia Pfizer Luxembourg SARL filial na Estônia Tel.: +372 666 7500 | Áustria Pfizer Corporation Austria Ges.m.b.H Tel: + 43 (0)1 521 15-0 |
Grécia Pfizer Ελλάς A.E. Tel.: +30 210 6785 800 | Polônia Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
Espanha Pfizer, S.L. Tel: +34914909900 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
França Pfizer Tel: +33 1 58 07 34 40 | Romênia Pfizer Romênia S.R.L Tel: +40 (0) 21 207 28 00 |
Croácia Pfizer Croácia d.o.o. Tel: + 385 1 3908 777 | Eslovênia Pfizer Luxembourg SARL Pfizer, filial para consultoria em farmacêutica Tel.: + 386 (0) 1 52 11 400 |
Irlanda Pfizer Healthcare Irlanda Unlimited Company Tel: 1800 633 363 (gratuito) +44 (0)1304 616161 | República Eslovaca Pfizer Luxembourg SARL, filial Tel: + 421 2 3355 5500 |
Islândia Icepharma hf Simi: + 354 540 8000 | Finlândia Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Itália Pfizer S.r.l. Tel: +39 06 33 18 21 | Suécia Pfizer AB Tel: +46 (0)8 550 520 00 |
Chipre Pfizer Ελλάς Α.Ε. (filial no Chipre) Tel: +357 22 817690 | |
Letônia Pfizer Luxembourg SARL filial na Letônia Tel.: + 371 670 35 775 |
Data da última revisão deste prospecto: 01/2025.
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu.
-------------------------------------------------------------------------------------------------------------------
Esta informação é destinada apenas a profissionais de saúde:
A vacina deve ser administrada apenas por via intramuscular. Não administrar por via intravascular, intradérmica ou subcutânea.
Se Nimenrix for administrado ao mesmo tempo que outras vacinas, devem ser utilizados locais de injeção diferentes.
Nimenrix não deve ser misturado com outras vacinas.
Instruções para a reconstituição da vacina com o diluente em seringa pré-carregada:
Nimenrix deve ser reconstituído adicionando todo o conteúdo da seringa pré-carregada ao frasco que contém o pó.
Para saber como inserir a agulha na seringa, veja o desenho explicativo. No entanto, a seringa fornecida com Nimenrix pode ser ligeiramente diferente (sem rosca de parafuso) da seringa descrita no desenho. Nesse caso, a agulha deve ser inserida sem parafusar.
|
|
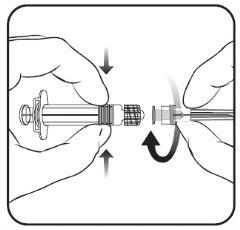
- Insira a agulha na seringa
e, em seguida, gire-a no sentido
das agulhas do relógio até que se bloqueie
(ver desenho).
- Retire o protetor da agulha;
 em algumas ocasiões pode
em algumas ocasiões pode
ser um pouco difícil.
- Adicione o diluente ao pó. Depois de adicionar o diluente ao pó, deve agitar bem a mistura até que o pó esteja completamente dissolvido no diluente.
A vacina reconstituída é uma solução transparente incolor.
Deve-se inspecionar visualmente o conteúdo da vacina reconstituída para observar se existe alguma substância estranha e/ou variação do aspecto físico antes de sua administração. Em caso de que se observe alguma dessas circunstâncias, descarte a vacina.
Após a reconstituição, a vacina deve ser administrada rapidamente.
Deve-se utilizar uma agulha nova para administrar a vacina.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a regulamentação local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a NIMENRIX pó e solvente para solução injetável em seringa pré-carregadaForma farmacêutica: INJETÁVEL, 0,5 mlFabricante: Sanofi Winthrop IndustrieRequer receita médicaForma farmacêutica: INJETÁVEL, 0,5 mlFabricante: Sanofi Winthrop IndustrieRequer receita médicaForma farmacêutica: INJETÁVEL, 10 microgramas de polissacarídeo do grupo A da Neisseria Meningitidis/dose - REVISAR µgFabricante: Sanofi Winthrop IndustrieRequer receita médica
Alternativas a NIMENRIX pó e solvente para solução injetável em seringa pré-carregada noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a NIMENRIX pó e solvente para solução injetável em seringa pré-carregada em Ukraina
Médicos online para NIMENRIX pó e solvente para solução injetável em seringa pré-carregada
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de NIMENRIX pó e solvente para solução injetável em seringa pré-carregada – sujeita a avaliação médica e regras locais.