LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL

Pergunte a um médico sobre a prescrição de LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL

Como usar LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL
Introdução
Prospecto:informação para o paciente adulto
Lucentis 10mg/ml solução injetável
ranibizumab
ADULTOS
Informação para bebês nascidos prematuramente no verso do prospecto.
Leia todo o prospecto atentamente antes deque lhe administrem este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico.
- Se experimentar efeitos adversos, consulte o seu médico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Lucentis e para que é utilizado
- O que necessita saber antes de que lhe administrem Lucentis
- Como é administrado Lucentis
- Posíveis efeitos adversos
- Conservação de Lucentis
- Conteúdo do envase e informações adicionais
1. O que é Lucentis e para que é utilizado
O que é Lucentis
Lucentis é uma solução que se injeta no olho. Lucentis pertence a um grupo de medicamentos denominados agentes anti-angiogénese. Contém o princípio ativo denominado ranibizumab.
Para que é utilizado Lucentis
Lucentis é utilizado em adultos para tratar várias doenças oculares que causam alteração da visão.
Estas doenças são o resultado de uma lesão na retina (camada sensível à luz na parte posterior do olho) provocada por:
- O crescimento de vasos sanguíneos anormais, que perdem líquido. Isto é observado em doenças como a degeneração macular associada à idade (DMAI) e a retinopatia diabética proliferativa (RDP, uma doença provocada pelo diabetes). Também pode estar associado à neovascularização coroide (NVC) devida à miopia patológica (MP), estrías angioides, corioretinopatia serosa central ou NVC inflamatória.
- Edema macular (inchação do centro da retina). A causa desta inchação pode ser o diabetes (uma doença conhecida como edema macular diabético (EMD)) ou um bloqueio das veias retinianas da retina (uma doença conhecida como oclusão da veia da retina (OVR)).
Como actua Lucentis
Lucentis reconhece e une-se de forma específica a uma proteína denominada factor de crescimento endotelial vascular A (VEGF-A) humano presente nos olhos. Em excesso, o VEGF-A causa o crescimento de vasos sanguíneos anormais e inchação no olho que pode ocasionar uma alteração da visão em doenças como DMAI, EMD, RDP, OVR, MP e NVC. Mediante a união ao VEGF-A, Lucentis pode impedir que actue e prevenir dito crescimento e inchação anormais.
Nestas doenças, Lucentis pode ajudar a estabilizar e, em muitos casos, melhorar a sua visão.
2. O que necessita saber antes de que lhe administrem Lucentis
Não lhe devem administrar Lucentis
- Se é alérgico ao ranibizumab ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
- Se tem uma infecção no olho ou à volta do mesmo.
- Se tem dor ou vermelhidão (inflamação intraocular grave) no olho.
Advertências e precauções
Consulte o seu médico antes de que lhe administrem Lucentis
- Lucentis é administrado mediante uma injeção no olho. Ocasionalmente, após o tratamento com Lucentis pode aparecer uma infecção na parte interna do olho, dor ou vermelhidão (inflamação), desprendimento ou desgarro de uma das camadas situadas no fundo do olho (desprendimento ou desgarro da retina e desprendimento ou desgarro do epitélio pigmentário da retina), ou enturbiação do cristalino (catarata). É importante identificar e tratar tal infecção ou desprendimento de retina o mais cedo possível. Informe imediatamente o seu médico se nota sinais como dor no olho ou aumento das molestias no olho, se piora a vermelhidão no olho, visão borrosa ou diminuição da visão, um aumento do número de pequenas manchas na visão ou aumento da sensibilidade à luz.
- Em alguns pacientes, após a injeção a pressão no olho pode aumentar durante um curto período de tempo. É possível que você não se aperceba disso, por isso pode que o seu médico lhe faça um seguimento da pressão ocular após cada injeção.
- Informe o seu médico se teve doenças nos olhos ou recebeu algum tratamento nos olhos anteriormente, ou se sofreu um acidente vascular cerebral ou teve sinais passageiros de acidente vascular cerebral (debilidade ou paralisia de um membro ou face, dificuldade na fala ou na compreensão). Esta informação será tida em consideração para avaliar se Lucentis é o tratamento apropriado para si.
Para consultar informações mais detalhadas sobre os efeitos adversos que poderiam ocorrer durante o tratamento com Lucentis, ver secção 4 (“Posíveis efeitos adversos”).
Crianças e adolescentes (menores de 18anos)
Excepto para a retinopatia do prematuro, não se recomenda o uso de Lucentis em crianças e adolescentes, pois não se estabeleceu nestes grupos etários. Para o tratamento de bebês nascidos prematuramente com retinopatia do prematuro (ROP) veja o verso do prospecto.
Outros medicamentos e Lucentis
Informe o seu médico se está utilizando, utilizou recentemente ou poderia ter que utilizar qualquer outro medicamento.
Gravidez e amamentação
- As mulheres que pudessem ficar grávidas devem utilizar um método anticonceptivo eficaz durante o tratamento e durante pelo menos três meses após a última injeção de Lucentis.
- Não há experiência no uso de Lucentis em mulheres grávidas. Lucentis não deve ser usado durante a gravidez salvo que o benefício potencial supere o risco potencial para o feto. Se está grávida, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico antes do tratamento com Lucentis.
- Pequenas quantidades de Lucentis podem passar para o leite materno, por isso não se recomenda o uso de Lucentis durante a amamentação. Consulte o seu médico ou farmacêutico antes do tratamento com Lucentis.
Condução e uso de máquinas
Após o tratamento com Lucentis você pode experimentar visão borrosa temporariamente. Se isso lhe acontecer, não conduza nem use máquinas até que este sintoma desapareça.
3. Como é administrado Lucentis
Lucentis é administrado pelo oftalmologista em forma de injeção única no olho sob anestesia local. A dose habitual de uma injeção é 0,05 ml (que contém 0,5 mg de princípio ativo). O intervalo entre duas doses aplicadas no mesmo olho deve ser como mínimo de quatro semanas. Todas as injeções serão administradas por um oftalmologista.
Para prevenir uma infecção, antes da injeção o seu médico lavará o olho cuidadosamente. O seu médico também lhe administrará um anestésico local para reduzir ou prevenir qualquer dor que possa sentir com a injeção.
O tratamento começa com uma injeção de Lucentis cada mês. O seu médico controlará a doença do seu olho e dependendo de como responde ao tratamento, decidirá se precisa ou não receber mais tratamento e quando precisa ser tratado.
No final do prospecto, na secção “Como preparar e administrar Lucentis em adultos” são dadas instruções detalhadas de uso.
Pacientes de idade avançada (65anos e mais)
Lucentis pode ser utilizado em pessoas de 65 anos de idade ou mais, e não é necessário um ajuste da dose.
Antes de interromper o tratamento com Lucentis
Se você está pensando em interromper o tratamento com Lucentis, vá à próxima consulta e comente antes com o seu médico. O seu médico aconselhará e decidirá durante quanto tempo deverá ser tratado com Lucentis.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Os efeitos adversos associados à administração de Lucentis devem-se ou ao próprio medicamento ou ao procedimento de injeção e a maioria afeta o olho.
A seguir são descritos os efeitos adversos mais graves:
Efeitos adversos graves frequentes(podem afetar até 1 de cada 10 pacientes): Desprendimento ou desgarro de uma camada na parte interna do olho (desprendimento ou desgarro da retina), que dá como resultado destelos de luz com partículas flutuantes que progridem para uma perda de visão transitoria ou para uma enturbiação do cristalino (catarata).
Efeitos adversos graves pouco frequentes(podem afetar até 1 de cada 100 pacientes): Cegueira, infecção do globo ocular (endoftalmite) com inflamação da parte interna do olho.
Os sintomas que poderia experimentar são dor ou aumento das molestias no olho, se piora a vermelhidão no olho, visão borrosa ou diminuição da visão, um aumento do número de pequenas manchas na visão ou aumento da sensibilidade à luz. Informe o seu médico imediatamente se apresentar algum destes efeitos adversos.
A seguir são descritos os efeitos adversos comunicados mais frequentemente:
Efeitos adversos muito frequentes(podem afetar mais de 1 de cada 10 pacientes)
Os efeitos adversos oculares incluem: Inflamação do olho, sangramento na parte posterior do olho (hemorragia na retina), alterações visuais, dor no olho, pequenas partículas ou manchas na visão (partículas flutuantes), sangue no olho, irritação do olho, sensação de ter algo dentro do olho, aumento da produção de lágrimas, inflamação ou infecção no bordo das pálpebras, olho seco, vermelhidão ou picor no olho e aumento da pressão no olho.
Os efeitos adversos não oculares incluem: Dor de garganta, congestão nasal, gotejamento nasal, dor de cabeça e dor nas articulações.
A seguir são descritos outros efeitos adversos que podem ocorrer após o tratamento com Lucentis:
Efeitos adversos frequentes
Os efeitos adversos oculares incluem: Diminuição da nitidez da visão, inchação de uma secção do olho (úvea, córnea), inflamação da córnea (parte frontal do olho), pequenas marcas na superfície do olho, visão borrosa, sangramento no local de injeção, sangramento no olho, secreção do olho com picor, vermelhidão e inchação (conjuntivite), sensibilidade à luz, molestias no olho, inchação da pálpebra, dor na pálpebra.
Os efeitos adversos não oculares incluem: Infecção das vias urinárias, contagem de glóbulos vermelhos baixa (com sintomas tais como cansaço, dificuldade ao respirar, tontura, palidez), ansiedade, tosse, náuseas, reações alérgicas tais como erupção, urticária, picor e vermelhidão da pele.
Efeitos adversos pouco frequentes
Os efeitos adversos oculares incluem: Inflamação e sangramento na parte anterior do olho, acúmulo de pus no olho, mudanças na parte central da superfície ocular, dor ou irritação no local de injeção, sensação anormal no olho, irritação da pálpebra.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Lucentis
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece na caixa após CAD e na etiqueta do frasco após EXP. A data de validade é o último dia do mês que se indica.
- Conservar em frigorífico (entre 2°C e 8°C). Não congelar.
- Antes de usar, o frasco sem abrir pode ser conservado a temperatura ambiente (25°C) durante um máximo de 24 horas.
- Conservar o frasco no embalagem exterior para protegê-lo da luz.
- Não utilize nenhum envase que esteja danificado.
6. Conteúdo do envase e informação adicional
Composição de Lucentis
- O princípio ativo é ranibizumab. Cada ml contém 10 mg de ranibizumab. Cada frasco contém 2,3 mg de ranibizumab em 0,23 ml de solução. Isso fornece uma quantidade adequada para fornecer uma dose única de 0,05 ml, que contém 0,5 mg de ranibizumab.
- Os demais componentes são α,α-trehalosa dihidrato; hidrocloruro de histidina monohidrato; histidina; polissorbato 20; água para injeção.
Aspecto do produto e conteúdo do envase
Lucentis é uma solução injetável contida em um frasco (0,23 ml). A solução é transparente, incolora a amarelo pardacento pálido e aquosa.
Existem dois tipos de embalagens diferentes:
Embalagem apenas com frasco
Embalagem que contém um frasco de vidro com ranibizumab, com tampa de borracha de clorobutilo. O frasco é para uso único.
Embalagem de frasco + agulha com filtro
Embalagem que contém um frasco de vidro com ranibizumab, com tampa de borracha de clorobutilo e uma agulha romba com filtro (18G x 1½", 1,2 mm x 40 mm, 5 micrómetros) para extrair o conteúdo do frasco. Todos os componentes são para uso único.
Título da autorização de comercialização
Novartis Europharm Limited
Edifício Vista
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsável pela fabricação
Novartis Farmacêutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Espanha
Lek Pharmaceuticals d.d.
Verovškova ulica 57
Ljubljana, 1526
Eslovênia
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Alemanha
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemanha
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lituânia SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxemburgo Novartis Pharma N.V. Tel: +32 2 246 16 11 |
República Checa Novartis s.r.o. Tel: +420 225 775 111 | Hungria Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Dinamarca Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Alemanha Novartis Pharma GmbH Tel: +49 911 273 0 | Países Baixos Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estônia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Noruega Novartis Norge AS Tel: +47 23 05 20 00 |
Grécia Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Áustria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Espanha Novartis Farmacêutica, S.A. Tel: +34 93 306 42 00 | Polônia Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
França Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croácia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romênia Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Irlanda Novartis Ireland Limited Tel: +353 1 260 12 55 | Eslovênia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Islândia Vistor hf. Tel: +354 535 7000 | República Eslovaca Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Itália Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finlândia Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Chipre Novartis Pharma Services Inc. Tel: +357 22 690 690 | Suécia Novartis Sverige AB Tel: +46 8 732 32 00 |
Letônia SIA Novartis Baltics Tel: +371 67 887 070 |
Data da última revisão deste prospecto:
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu
Esta informação é destinada apenas a profissionais de saúde:
Ver também a seção 3 “Como administrar Lucentis”.
Como preparar e administrar Lucentis em adultos
Frasco para uso único. Apenas para via intravítrea.
Lucentis deve ser administrado por um oftalmologista com experiência na administração de injeções intravítreas.
Na DMAE exsudativa, na NVC, na RDP e na alteração visual devido à EMD ou ao edema macular secundário à OVR, a dose recomendada de Lucentis é de 0,5 mg administrada em forma de injeção intravítrea única. Isso corresponde a um volume de injeção de 0,05 ml. O intervalo entre duas doses injetadas no mesmo olho deve ser de pelo menos quatro semanas.
O tratamento é iniciado com uma injeção por mês até alcançar a acuidade visual máxima e/ou não haja sinais de atividade da doença, ou seja, nenhum cambio na acuidade visual ou em outros sinais e sintomas da doença sob tratamento contínuo. Em pacientes com DMAE exsudativa, EMD, RDP e OVR, inicialmente podem ser necessárias três ou mais injeções consecutivas administradas mensalmente.
A partir desse momento, os intervalos de monitorização e tratamento devem ser determinados de acordo com o critério médico e com base na atividade da doença, avaliada mediante a acuidade visual e/ou parâmetros anatômicos.
Deve-se interromper o tratamento com Lucentis se, sob critério do médico, os parâmetros visuais e anatômicos indicam que o paciente não está se beneficiando do tratamento contínuo.
A monitorização para determinar a atividade da doença pode incluir exame clínico, controle funcional ou técnicas de imagem (p. ex. tomografia de coerência óptica ou angiografia com fluoresceína).
Se os pacientes estiverem sendo tratados de acordo com um regime de tratar e estender, uma vez que a acuidade visual máxima tenha sido alcançada e/ou não haja sinais de atividade da doença, os intervalos de tratamento podem ser espaçados gradualmente até que os sinais de atividade da doença ou alteração visual voltem a aparecer. No caso da DMAE exsudativa, o intervalo de tratamento não deve ser espaçado em mais de duas semanas cada vez, e no caso do EMD, pode ser espaçado até um mês cada vez. Para a RDP e a OVR, os intervalos de tratamento também podem ser espaçados gradualmente, no entanto, os dados disponíveis não são suficientes para determinar a duração desses intervalos. Se a atividade da doença voltar a aparecer, o intervalo de tratamento deve ser encurtado de forma consecutiva.
O tratamento da alteração visual devido à NVC deve ser determinado para cada paciente de forma individualizada com base na atividade da doença. Alguns pacientes podem precisar apenas de uma injeção durante os primeiros 12 meses; outros podem precisar de tratamento com mais frequência, incluindo uma injeção mensal. No caso de NVC secundária à miopia patológica (MP), muitos pacientes podem precisar apenas de uma ou duas injeções durante o primeiro ano.
Lucentis e fotocoagulação com laser em EMD e edema macular secundário à oclusão da ramo venosa retiniana (ORVR)
Existe alguma experiência com Lucentis administrado concomitantemente com fotocoagulação com laser. Quando administrados no mesmo dia, Lucentis deve ser administrado pelo menos 30 minutos após a fotocoagulação com laser. Lucentis pode ser administrado em pacientes que receberam fotocoagulação com laser previamente.
Lucentis e a terapia fotodinâmica com verteporfina na NVC secundária à MP
Não há experiência na administração concomitante de Lucentis e verteporfina.
Antes da administração de Lucentis, deve-se verificar visualmente a ausência de partículas e decoloração.
O procedimento de injeção deve ser realizado sob condições assépticas, que incluem o lavado quirúrgico das mãos, o uso de luvas estéreis, um campo estéril, um blefarostato estéril para as pálpebras (ou equivalente) e a disponibilidade de uma paracentese estéril (se necessário). Antes de realizar o procedimento de injeção intravítrea, deve-se avaliar detalhadamente a história clínica do paciente em relação a reações de hipersensibilidade. Antes da injeção, deve-se administrar uma anestesia adequada e um microbicida tópico de amplo espectro para desinfetar a pele da zona periocular, pálpebra e superfície ocular, de acordo com a prática local.
Embalagem apenas com frasco
O frasco é para uso único. Após a injeção, deve-se descartar qualquer sobra de produto não utilizado. Não se deve usar nenhum frasco que mostre sinais de deterioração ou manipulação. A esterilidade só pode ser garantida se o selo do envase for mantido intacto.
Para a preparação e a injeção intravítrea, são necessários os seguintes produtos sanitários (para uso único):
- uma agulha com filtro de 5 µm (18G)
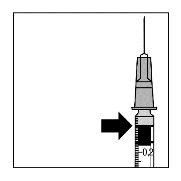
- uma seringa estéril de 1 ml (que inclua uma marca em 0,05 ml)
- uma agulha para injeção (30G x ½").
Esses produtos sanitários não estão incluídos na embalagem de Lucentis.
Embalagem de frasco + agulha com filtro
Todos os componentes são estéreis e para uso único. Não se deve usar nenhum componente cujo envase mostre sinais de deterioração ou manipulação. A esterilidade só pode ser garantida se o selo do envase dos componentes for mantido intacto. A reutilização pode causar uma infecção ou outra doença/lesão.
Para a preparação e a injeção intravítrea, são necessários os seguintes produtos sanitários (para uso único):
- uma agulha com filtro de 5 µm (18G x 1½", 1,2 mm x 40 mm, fornecida)
- uma seringa estéril de 1 ml (que inclua uma marca em 0,05 ml, não incluída na embalagem de Lucentis)
- uma agulha para injeção (30G x ½", não incluída na embalagem de Lucentis)
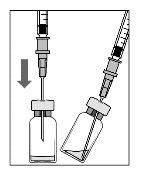
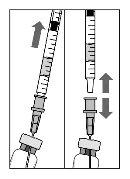
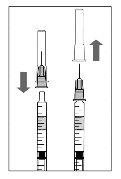
Para a preparação de Lucentis para administração intravítrea em pacientes adultos, siga as seguintes instruções:
|
|
|
|
|
Nota: Segure a agulha para injeção pelo cone enquanto retira a cápsula de fechamento. |
|
Nota:Não seque a agulha para injeção. Não puxe o êmbolo para trás. |
A agulha para injeção deve ser introduzida 3,5-4,0 mm por trás do limbo na cavidade vítrea, evitando o meridiano horizontal e em direção ao centro do globo. Em seguida, deve-se liberar o volume de injeção de 0,05 ml; as injeções subsequentes devem ser aplicadas cada vez em um ponto escleral distinto.
Após a injeção, não cubra a agulha com a cápsula de fechamento nem separe-a da seringa. Elimine a seringa usada junto com a agulha em um contenedor para objetos pontiagudos ou elimine de acordo com a regulamentação local.
Bula:informação para os tutores de bebês nascidos prematuramente
Lucentis 10mg/ml solução injetável
ranibizumab
BEBÊS NASCIDOS PREMATURAMENTE
Informação para adultos na outra face da bula.
Leia todo o prospecto atentamente antes deque lhe administrem este medicamento ao seu bebê, porque contém informações importantes para você.
- Conserva este prospecto, pois pode ter que relê-lo.
- Se tiver alguma dúvida, consulte o médico do seu bebê.
- Se o seu bebê experimentar efeitos adversos, consulte o médico do seu bebê, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Lucentis e para que é utilizado
- O que precisa saber antes de que lhe administrem Lucentis ao seu bebê
- Como administrar Lucentis
- Posíveis efeitos adversos
- Conservação de Lucentis
- Conteúdo do envase e informação adicional
- O que é Lucentis e para que é utilizado
O que é Lucentis
Lucentis é uma solução que se injeta no olho. Lucentis pertence a um grupo de medicamentos denominados agentes antineovascularização. Contém o princípio ativo denominado ranibizumab.
Para que é utilizado Lucentis
Lucentis é utilizado em bebês nascidos prematuramente para tratar a retinopatia do prematuro (ROP), uma doença que causa alteração da visão devido a uma lesão da parte posterior do olho (a retina) causada por um crescimento anormal dos vasos sanguíneos.
Como age Lucentis
Lucentis reconhece e se une de forma específica a uma proteína denominada fator de crescimento endotelial vascular A (VEGF-A) humano presente nos olhos. Em excesso, o VEGF-A causa o crescimento de vasos sanguíneos anómalos no olho. Lucentis pode impedir que atue e prevenir esse crescimento anormal.
- O que precisa saber antes de que lhe administrem Lucentis ao seu bebê
Não lhe devem administrar Lucentis ao seu bebê
- Se o seu bebê é alérgico ao ranibizumab ou a algum dos demais componentes deste medicamento (incluídos na seção 6).
- Se o seu bebê tem uma infecção no olho ou ao redor do mesmo.
- Se o seu bebê tem dor ou vermelhidão (inflamação intraocular grave) no olho.
Advertências e precauções
Consulte o médico do seu bebê antes de que lhe administrem Lucentis ao seu bebê
- Lucentis é administrado mediante uma injeção no olho. Ocasionalmente, após o tratamento com Lucentis, pode aparecer uma infecção na parte interna do olho, dor ou vermelhidão (inflamação), desprendimento ou desgarro de uma das camadas situadas no fundo do olho (desprendimento ou desgarro da retina e desprendimento ou desgarro do epitelio pigmentário da retina), ou enturbiação do cristalino (catarata). É importante identificar e tratar tal infecção ou desprendimento de retina o mais rápido possível. Informe imediatamente o médico se o seu bebê apresentar sinais como dor no olho ou se piorar a vermelhidão no olho.
Em alguns pacientes, após a injeção, a pressão no olho pode aumentar durante um curto período de tempo. O médico do seu bebê pode realizar um acompanhamento da pressão ocular após cada injeção.
Para consultar informações mais detalhadas sobre os efeitos adversos que podem ocorrer durante o tratamento com Lucentis, ver seção 4 (“Posíveis efeitos adversos”).
Outros medicamentos e Lucentis
Informe o médico do seu bebê se o seu bebê está tomando, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
- Como administrar Lucentis
Lucentis é administrado pelo oftalmologista em forma de injeção única nos olhos do seu bebê, normalmente sob anestesia local. A dose habitual de uma injeção é de 0,02 ml (que contém 0,2 mg de princípio ativo). O intervalo entre duas doses aplicadas no mesmo olho deve ser de pelo menos quatro semanas. Todas as injeções serão administradas pelo oftalmologista.
Para prevenir uma infecção, antes da injeção, o médico do seu bebê lavará os olhos do seu bebê cuidadosamente. O médico também administrará um anestésico local ao seu bebê para reduzir ou prevenir qualquer dor.
O tratamento é iniciado com uma injeção de Lucentis em cada olho (alguns bebês podem precisar de tratamento em apenas um olho). O médico controlará a doença do(s) olho(s) do seu bebê e, dependendo de como o seu bebê responde ao tratamento, decidirá se precisa ou não receber mais tratamento e quando precisa ser tratado.
No final da bula, na seção “Como preparar e administrar Lucentis em recém-nascidos prematuros”, são fornecidas instruções detalhadas de uso.
Antes de interromper o tratamento com Lucentis
Se você está considerando interromper o tratamento com Lucentis no seu bebê, vá à próxima consulta e comente antes com o médico do seu bebê. O médico do seu bebê o aconselhará e decidirá de acordo.
Durante quanto tempo o seu bebé deverá ser tratado com Lucentis.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao médico do seu bebé.
- Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Os efeitos adversos associados à administração de Lucentis devem-se ou ao próprio medicamento ou ao procedimento de injeção e a maioria afeta o olho.
A seguir se descrevem os efeitos adversos mais frequentes em bebés nascidos prematuramente:
Os efeitos adversos oculares incluem: sangramento na parte posterior do olho (sangramento da retina), sangramento no olho ou no local da injeção e sangue no olho (sangramento da conjuntiva).
Os efeitos adversos não oculares incluem: dor de garganta, congestão nasal e secreção nasal, baixo número de glóbulos vermelhos (com sintomas como cansaço, dificuldade respiratória e pele pálida), tosse, infecção do trato urinário, reações alérgicas como erupção e vermelhidão da pele.
A seguir se listam efeitos adversos adicionais, que se observaram com Lucentis em adultos. Estes efeitos adversos também podem ocorrer em bebés nascidos prematuramente.
A seguir se descrevem os efeitos adversos mais graves em adultos:
Efeitos adversos graves frequentes (podem afetar até 1 de cada 10 doentes): Desprendimento ou desgarro de uma camada na parte interna do olho (desprendimento ou desgarro da retina) que progride para uma perda de visão transitória ou um enturbiação do cristalino (catarata).
Efeitos adversos graves pouco frequentes (podem afetar até 1 de cada 100 doentes): Cegueira, infecção do globo ocular (endoftalmite) com inflamação da parte interna do olho.
É importante identificar e tratar o mais cedo possível os efeitos adversos graves, como a infecção do globo ocular ou o desprendimento de retina. Informar o médico imediatamente se o seu bebé apresentar sinais como dor nos olhos ou piora do vermelho dos olhos.
A seguir se descrevem os efeitos adversos notificados mais frequentemente em adultos:
Efeitos adversos muito frequentes (podem afetar mais de 1 de cada 10 doentes)
Os efeitos adversos oculares incluem: Inflamação do olho, alterações visuais, dor no olho, pequenas partículas ou manchas na visão (partículas flutuantes), irritação do olho, sensação de ter algo dentro do olho, aumento da produção de lágrimas, inflamação ou infecção no bordo das pálpebras, olho seco, vermelhidão ou coceira no olho e aumento da pressão no olho.
Os efeitos adversos não oculares incluem: Dor de cabeça e dor nas articulações.
Efeitos adversos frequentes
Os efeitos adversos oculares incluem: Diminuição da nitidez da visão, inchaço de uma seção do olho (úvea, córnea), inflamação da córnea (parte frontal do olho), pequenas marcas na superfície do olho, visão borrosa, secreção do olho com coceira, vermelhidão e inchaço (conjuntivite), sensibilidade à luz, molestias no olho, inchaço da pálpebra, dor na pálpebra.
Os efeitos adversos não oculares incluem: Ansiedade, náuseas.
Efeitos adversos pouco frequentes
Os efeitos adversos oculares incluem: Inflamação e sangramento na parte anterior do olho, acúmulo de pus no olho, alterações na parte central da superfície ocular, dor ou irritação no local da injeção, sensação anormal no olho, irritação da pálpebra.
Se tiver alguma dúvida sobre os efeitos adversos, pergunte ao médico do seu bebé.
Comunicação de efeitos adversos
Se o seu bebé experimentar qualquer tipo de efeito adverso, consulte o médico do seu bebé, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
- Conservação de Lucentis
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece na caixa após CAD e na etiqueta do frasco após EXP. A data de validade é o último dia do mês que se indica.
- Conservar em frigorífico (entre 2°C e 8°C). Não congelar.
- Antes de usar, o frasco sem abrir pode ser conservado a temperatura ambiente (25°C) durante um máximo de 24 horas.
- Conservar o frasco no embalagem exterior para protegê-lo da luz.
- Não utilize nenhum recipiente que esteja danificado.
- Conteúdo do embalagem e informações adicionais
Composição de Lucentis
- O princípio ativo é ranibizumab. Cada ml contém 10 mg de ranibizumab. Cada frasco contém 2,3 mg de ranibizumab em 0,23 ml de solução. Isso fornece uma quantidade adequada para fornecer uma dose única de 0,02 ml, que contém 0,2 mg de ranibizumab.
- Os demais componentes são α,α-trehalosa dihidrato; hidrocloruro de histidina monohidrato; histidina; polissorbato 20; água para injeção.
Aspecto do produto e conteúdo do embalagem
Lucentis é uma solução injetável contida em um frasco (0,23 ml). A solução é transparente, incolora a amarelo pálido aquoso.
Existem dois tipos de embalagens diferentes:
Embalagem apenas com frasco
Embalagem que contém um frasco de vidro com ranibizumab, com tampão de borracha de clorobutilo. O frasco é para uso único.
Embalagem de frasco + agulha com filtro
Embalagem que contém um frasco de vidro com ranibizumab, com tampão de borracha de clorobutilo e uma agulha roma com filtro (18G x 1½″, 1,2 mm x 40 mm, 5 micrómetros) para extrair o conteúdo do frasco. Todos os componentes são para uso único.
Título da autorização de comercialização
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsável pela fabricação
Novartis Farmacêutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Espanha
Lek Pharmaceuticals d.d.
Verovškova ulica 57
Ljubljana, 1526
Eslovênia
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Alemanha
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemanha
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lituânia SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxemburgo Novartis Pharma N.V. Tel: +32 2 246 16 11 |
República Checa Novartis s.r.o. Tel: +420 225 775 111 | Hungria Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Dinamarca Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Alemanha Novartis Pharma GmbH Tel: +49 911 273 0 | Países Baixos Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estônia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Noruega Novartis Norge AS Tel: +47 23 05 20 00 |
Grécia Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Áustria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Espanha Novartis Farmacêutica, S.A. Tel: +34 93 306 42 00 | Polônia Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
França Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croácia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romênia Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Irlanda Novartis Ireland Limited Tel: +353 1 260 12 55 | Eslovênia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Islândia Vistor hf. Tel: +354 535 7000 | República Eslovaca Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Itália Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finlândia Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Chipre Novartis Pharma Services Inc. Tel: +357 22 690 690 | Suécia Novartis Sverige AB Tel: +46 8 732 32 00 |
Letônia SIA Novartis Baltics Tel: +371 67 887 070 |
Data da última revisão deste prospecto:
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu
Esta informação está destinada apenas a profissionais de saúde:
Ver também a seção 3 “Como se administra Lucentis”.
Como preparar e administrar Lucentis em recém-nascidos pré-termo
Frasco para uso único. Apenas para via intravítrea.
Lucentis deve ser administrado por um oftalmologista que tenha experiência na administração de injeções intravítreas em recém-nascidos pré-termo.
Para o tratamento de recém-nascidos pré-termo, use a seringa de baixo volume e alta precisão que se fornece com uma agulha para injeção (30G x ½″) no kit VISISURE.
Para os recém-nascidos pré-termo, a dose recomendada de Lucentis é 0,2 mg administrada em forma de injeção intravítrea única.Isso corresponde a um volume de injeção de 0,02 ml. Nos recém-nascidos pré-termo, o tratamento da retinopatia do prematuro (ROP) é iniciado com uma única injeção por olho e pode ser administrado bilateralmente no mesmo dia. No total, podem ser administradas até três injeções em cada olho dentro dos seis meses posteriores ao início do tratamento se apresentarem sinais de atividade da doença. No ensaio clínico RAINBOW de 24 semanas de duração, a maioria dos pacientes (78%) recebeu uma injeção por olho. Os pacientes que foram tratados com 0,2 mg neste ensaio clínico não necessitaram de tratamento adicional no posterior estudo de extensão a longo prazo que seguiu os pacientes até os cinco anos de idade. Não foi estudada a administração de mais de três injeções por olho. O intervalo entre duas doses injetadas no mesmo olho deve ser de pelo menos quatro semanas.
Antes da administração de Lucentis, deve-se verificar visualmente a ausência de partículas e decoloração.
O procedimento de injeção deve ser realizado sob condições assépticas, que incluem o lavado cirúrgico das mãos, o uso de luvas estéreis, um campo estéril, um blefarostato estéril para as pálpebras (ou equivalente) e a disponibilidade de uma paracentese estéril (se necessário). Antes de realizar o procedimento de injeção intravítrea, deve-se avaliar detalhadamente a história clínica do paciente em relação a reações de hipersensibilidade. Antes da injeção, deve-se administrar uma anestesia adequada e um microbicida tópico de amplo espectro para desinfetar a pele da zona periocular, pálpebra e superfície ocular, de acordo com a prática local.
Embalagem apenas com frasco
O frasco é para uso único. Após a injeção, deve-se descartar qualquer sobra de produto não utilizado. Não se deve utilizar nenhum frasco que mostre sinais de deterioração ou manipulação. A esterilidade só pode ser garantida se o selo do embalagem se mantém intacto.
Para a preparação e a injeção intravítrea, são necessários os seguintes produtos sanitários (para uso único):
- uma agulha com filtro de 5 µm (18G); não está incluída no embalagem de Lucentis
- uma seringa estéril de alta precisão e baixo volume (fornecida separadamente dentro do kit VISISURE)
- uma agulha para injeção (30G x ½″); (fornecida separadamente dentro do kit VISISURE).
Embalagem de frasco + agulha com filtro
Todos os componentes são estéreis e para uso único. Não se deve utilizar nenhum componente cujo embalagem mostre sinais de deterioração ou manipulação. A esterilidade só pode ser garantida se o selo do embalagem dos componentes se mantém intacto. A reutilização pode dar origem a uma infecção ou outra doença/lesão.
Para a preparação e a injeção intravítrea, são necessários os seguintes produtos sanitários (para uso único):
- uma agulha com filtro de 5 µm (18G x 1½″, 1,2 mm x 40 mm, 5 micrómetros) fornecida
- uma seringa estéril de alta precisão e baixo volume (fornecida separadamente dentro do kit VISISURE)
- uma agulha para injeção (30G x ½″); (fornecida separadamente dentro do kit VISISURE)
Para preparar Lucentis para a administração intravítrea em recém-nascidos pré-termo, siga as instruções de uso do kit VISISURE.
A agulha para injeção deve ser introduzida 1,0 a 2,0 mm por trás do limbo na cavidade vítrea, com a agulha em direção ao nervo óptico. Em seguida, deve-se liberar o volume de injeção de 0,02 ml.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVELForma farmacêutica: INJETÁVEL, 10 mg/mLSubstância ativa: ranibizumabFabricante: Samsung Bioepis Nl B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 10 mg/mLSubstância ativa: ranibizumabFabricante: Novartis Europharm LimitedRequer receita médicaForma farmacêutica: INJETÁVEL, 10 mg/mlSubstância ativa: ranibizumabFabricante: Midas Pharma GmbhRequer receita médica
Alternativas a LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL em Украина
Médicos online para LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de LUCENTIS 10 mg/ml SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.