
LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO

Como usar LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o paciente
Levetiracetam SUN 100 mg/ml concentrado para solução para perfusão EFG
Levetiracetam
Leia todo o prospecto detenidamente antes de você ou seu filho começar a usar este medicamento, porque contém informações importantes para você.
- Conserva este prospecto, pois pode ter que relê-lo.
- Se tiver alguma dúvida, consulte seu médico ou enfermeiro.
- Se experimentar efeitos adversos, consulte seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto:
- O que é Levetiracetam SUN e para que se utiliza
- O que precisa saber antes de começar a usar Levetiracetam SUN
- Como usar Levetiracetam SUN
- Possíveis efeitos adversos
- Conservação de Levetiracetam SUN
- Conteúdo do envase e informações adicionais
1. O que é Levetiracetam SUN e para que se utiliza
Levetiracetam é um medicamento antiepiléptico (um medicamento para o tratamento de crises na epilepsia).
Levetiracetam SUN é utilizado:
- em solitário em adultos e adolescentes de 16 anos de idade ou mais com epilepsia diagnosticada recentemente para tratar uma forma de epilepsia. A epilepsia é uma doença onde os pacientes têm ataques (crises). Levetiracetam é utilizado para a forma de epilepsia na qual as crises inicialmente afetam apenas um lado do cérebro, mas podem depois estender-se a zonas mais amplas nos dois lados do cérebro (crises de início parcial com ou sem generalização secundária). Seu médico lhe prescreveu levetiracetam para reduzir o número de crises.
- conjuntamente com outros medicamentos antiepilépticos (terapia concomitante) para tratar:
- as crises de início parcial com ou sem generalização em adultos, adolescentes e crianças a partir de 4 anos de idade.
- as crises mioclônicas (sacudidas tipo choque, curtas, de um músculo ou grupo de músculos) em adultos e adolescentes a partir de 12 anos com epilepsia mioclônica juvenil.
- as crises tônico-clônicas generalizadas primárias (crises maiores, incluindo perda de consciência) em adultos e adolescentes a partir de 12 anos de idade com epilepsia idiopática generalizada (tipo de epilepsia que se pensa que tem uma causa genética).
Levetiracetam SUN concentrado pode ser utilizado quando a administração oral de Levetiracetam SUN é temporariamente inviável.
2. O que precisa saber antes de começar a usar Levetiracetam SUN
Não use Levetiracetam SUN
- Se é alérgico a levetiracetam, aos derivados de pirrolidona ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6).
Advertências e precauções
Consulte seu médico antes de começar a usar Levetiracetam SUN
- Se você padece problemas de rim, siga as instruções de seu médico, que decidirá se deve ajustar a dose a tomar.
- Se observa qualquer diminuição no crescimento de seu filho ou se se produz um desenvolvimento da puberdade inesperado, entre em contato com seu médico.
- Um pequeno número de pessoas em tratamento com antiepilépticos, tais como Levetiracetam SUN, tiveram pensamentos de se fazerem mal ou se suicidarem. Se você tem qualquer sintoma de depressão e/ou pensamentos suicidas, entre em contato com seu médico.
- Se você tem antecedentes médicos ou familiares de ritmo cardíaco irregular (visível em eletrocardiograma), ou se você tem uma doença e/ou toma um tratamento que o faça propenso a arritmias cardíacas ou desequilíbrios de sais.
Informe seu médico ou farmacêutico se algum dos seguintes efeitos adversos se agrava ou dura mais de alguns dias:
- pensamentos anormais, sensação de irritabilidade ou reação de forma mais agressiva do que o normal ou se você ou sua família e amigos notam mudanças importantes no estado de ânimo ou comportamento
- agravamento da epilepsia
Em raros casos, as crises epilépticas podem piorar ou ocorrer com mais frequência, principalmente durante o primeiro mês após o início do tratamento ou do aumento da dose.
Em uma forma muito rara de epilepsia de início precoce (epilepsia associada a mutações SCN8A) que causa múltiplos tipos de crises epilépticas e perda de habilidades, você pode notar que as crises continuam presentes ou pioram durante o tratamento.
Se você experimenta algum desses sintomas novos enquanto toma Levetiracetam SUN, procure um médico o mais rápido possível.
Crianças e adolescentes
O tratamento exclusivo com Levetiracetam SUN (monoterapia) não é indicado em crianças e adolescentes menores de 16 anos.
Uso de Levetiracetam SUN com outros medicamentos
Informe seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento.
Não tome macrogol (medicamento utilizado como laxante) durante uma hora antes e uma hora após tomar levetiracetam, pois pode reduzir seu efeito.
Gravidez e lactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte seu médico antes de utilizar este medicamento. Levetiracetam somente pode ser utilizado durante a gravidez se, após uma cuidadosa avaliação, seu médico o considerar necessário.
Não deve abandonar seu tratamento sem comentar antes com seu médico.
Não se pode excluir completamente o risco de defeitos de nascimento para o bebê.
Não se recomenda a lactação natural durante o tratamento.
Condução e uso de máquinas
Levetiracetam SUN pode alterar sua capacidade para conduzir ou manejar ferramentas ou máquinas, pois pode produzir sensação de sono. Isso é mais provável no início do tratamento ou quando se aumenta a dose. Não deve conduzir ou utilizar máquinas até que se verifique que sua capacidade para realizar essas atividades não está afetada.
Levetiracetam SUN contém sódio
Os outros componentes são acetato de sódio tri-hidratado, ácido acético glacial, cloreto de sódio, água para preparações injetáveis. Uma dose unitária máxima de Levetiracetam SUN concentrado contém 2,5 mmol (ou 57 mg) de sódio (0,8 mmol (ou 19 mg) de sódio por frasco). Deve ser considerado em pacientes que sigam uma dieta baixa em sódio.
3. Como usar Levetiracetam SUN
Um médico ou um enfermeiro/a lhe administrará Levetiracetam SUN por meio de perfusão intravenosa. Levetiracetam SUN deve ser administrado duas vezes ao dia, uma vez pela manhã e outra à noite, aproximadamente à mesma hora todos os dias.
A formulação intravenosa é uma alternativa à administração oral. Pode passar de uma para outra sem cambiar a dose. Sua dose diária total e frequência de administração devem ser idênticas.
Terapia concomitante e monoterapia (a partir de 16 anos de idade)
Adultos (≥ 18 anos) e adolescentes (a partir de 12 a 17 anos) com um peso de 50 kg ou superior:
Quando começar a tomar Levetiracetam SUN, seu médico lhe prescreverá uma dose inferiordurante duas semanas antes de administrar a dose diária mais baixa.
Dose recomendada: entre 1.000 mg e 3.000 mg ao dia.
Dose em crianças (de 4 a 11 anos) e adolescentes (de 12 a 17 anos) com um peso inferior a 50 kg:
Dose recomendada: entre 20 mg por kg de peso corporal e 60 mg por kg de peso corporal ao dia.
Método e forma de administração:
Levetiracetam SUN é para administração intravenosa.
A dose recomendada deve ser diluída no mínimo em 100 ml de um diluente compatível e administrada por perfusão intravenosa durante 15 minutos.
É fornecida uma informação mais detalhada para o correto uso de Levetiracetam SUN na seção 6 para médicos e enfermeiros/as.
Duração do tratamento:
Não se tem experiência na administração de levetiracetam intravenoso por um período superior a 4 dias.
Se interromper o tratamento com Levetiracetam SUN:
Assim como acontece com outros medicamentos antiepilépticos, a finalização do tratamento com Levetiracetam SUN deve ser feita de forma gradual para evitar um aumento das crises. Se seu médico decidir parar seu tratamento com Levetiracetam SUN, lhe dará as instruções para a retirada gradual de Levetiracetam SUN, se decidir suspender seu tratamento com este medicamento.
Se você tiver alguma outra dúvida sobre o uso deste medicamento, pergunte a seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informe seu médico imediatamente, ou vá ao serviço de urgências do seu hospital mais próximo se você experimentar:
- fraqueza, tontura ou dificuldade para respirar, pois esses podem ser sinais de uma reação alérgica (anafilática) grave
- inchaço do rosto, lábios, língua ou garganta (edema de Quincke)
- sintomas de gripe e erupção no rosto seguido de uma erupção prolongada com temperatura elevada, níveis de enzimas hepáticas elevados em testes sanguíneos e um aumento em um tipo de células brancas sanguíneas (eosinofilia), nódulos linfáticos aumentados e a afetação de outros órgãos do corpo (Reação de hipersensibilidade ao medicamento com eosinofilia e sintomas sistémicos (DRESS))
- sintomas como baixo volume de urina, cansaço, náuseas, vômitos, confusão e inchaço de pernas, braços ou pés, pois pode ser um sinal de diminuição súbita da função renal
- erupção cutânea que pode dar lugar a bolhas que podem aparecer como pequenas dianas (pontos centrais escuros rodeados por uma área mais pálida, com um anel escuro ao redor da borda) (eritema multiforme)
- erupção generalizada com bolhas e descamação da pele, especialmente ao redor da boca, nariz, olhos e genitais (síndrome de Stevens-Johnson)
- forma mais grave que causa descamação da pele em mais de 30% da superfície corporal (necrólise epidérmica tóxica)
- sinais de mudanças mentais graves ou se alguém ao seu redor nota sinais de confusão, sonolência (adormecimento), amnésia (perda de memória), deterioração da memória (esquecimento), comportamento anormal ou outros sinais neurológicos, incluindo movimentos involuntários ou incontrolados. Esses podem ser sintomas de encefalopatia.
Os efeitos adversos notificados mais frequentemente são nasofaringite, sonolência (sensação de sono), dor de cabeça, fadiga e tontura. Os efeitos adversos como sensação de sono, sensação de fraqueza e tonturas podem ser mais frequentes quando se inicia o tratamento ou se aumenta a dose. No entanto, esses efeitos adversos devem diminuir com o tempo.
Muito frequentes:podem afetar mais de 1 de cada 10 pessoas
- nasofaringite;
- sonolência (sensação de sono), dor de cabeça.
Frequentes:podem afetar até 1 de cada 10 pessoas
- anorexia (perda de apetite);
- depressão, hostilidade ou agressividade, ansiedade, insônia, nervosismo ou irritabilidade;
- convulsões, distúrbio do equilíbrio, tonturas (sensação de instabilidade), letargia (falta de energia e entusiasmo), tremor (tremor involuntário);
- vértebra (sensação de rotação);
- tosse;
- dor abdominal, diarreia, dispepsia (digestão pesada, ardor e acidez), vômitos, náuseas;
- erupção na pele;
- astenia/fadiga (sensação de fraqueza).
Pouco frequentes: podem afetar até 1 de cada 100 pessoas
- diminuição do número de plaquetas, diminuição dos glóbulos brancos;
- perda de peso, aumento de peso;
- tentativa de suicídio e pensamentos suicidas, alterações mentais, comportamento anormal, alucinações, cólera, confusão, ataque de pânico, instabilidade emocional/mudanças de humor, agitação;
- amnésia (perda de memória), deterioração da memória (falta de memória), coordenação anormal/ataxia (coordenação dos movimentos alterada), parestesia (formigamento), alterações da atenção (perda de concentração);
- diplopia (visão dupla), visão borrosa;
- valores elevados/anormais nos testes sobre a funcionalidade do fígado;
- perda de cabelo, eczema, coceira;
- fraqueza muscular, mialgia (dor muscular);
- lesão.
Raros:podem afetar até 1 de cada 1.000 pessoas
- infecção;
- diminuição de todos os tipos de células sanguíneas;
- reações alérgicas graves (DRESS, reação anafilática (reação alérgica importante e grave), edema de Quincke (inchaço do rosto, lábios, língua e garganta));
- diminuição da concentração de sódio no sangue;
- suicídio, distúrbios da personalidade (problemas de comportamento), pensamento anormal (pensamento lento, dificuldade para se concentrar);
- delírios;
- encefalopatia (ver subseção “Informe seu médico imediatamente” para ver uma descrição detalhada dos sintomas);
- as crises epilépticas podem piorar ou ocorrer com mais frequência;
- espasmos musculares incontroláveis que afetam a cabeça, o torso e as extremidades, dificuldade para controlar os movimentos, hipercinesia (hiperatividade);
- mudança do ritmo cardíaco (eletrocardiograma)
- pancreatite (inflamação do pâncreas);
- insuficiência hepática, hepatite (inflamação do fígado);
- diminuição súbita da função renal;
- erupção cutânea, que pode dar lugar a bolhas que podem aparecer como pequenas dianas (pontos centrais escuros rodeados por uma área mais pálida, com um anel escuro ao redor da borda) (eritema multiforme), uma erupção generalizada com bolhas e descamação da pele, especialmente ao redor da boca, nariz, olhos e genitais (síndrome de Stevens-Johnson) e uma forma mais grave que causa descamação da pele em mais de 30% da superfície corporal (necrólise epidérmica tóxica);
- rabdomiólise (rotura do tecido muscular) e aumento de creatina fosfoquinase sanguínea associado. A prevalência é significativamente maior em pacientes japoneses em comparação com pacientes não japoneses
- coxeira ou dificuldade para caminhar.
- combinação de febre, rigidez muscular, pressão arterial e frequência cardíaca instáveis, confusão, estado de baixo nível de consciência (podem ser sinais de um distúrbio chamado síndrome neuroléptico maligno). A prevalência é significativamente maior em pacientes japoneses em comparação com pacientes não japoneses.
Muito raros: podem afetar até 1 de cada 10.000 pessoas
- pensamentos ou sensações não desejadas e repetidas ou o impulso de fazer algo uma e outra vez (distúrbio obsessivo-compulsivo).
Comunicação de efeitos adversos
Se você experimentar qualquer tipo de efeito adverso, consulte seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente por meio do sistema nacional de notificação incluído no Anexo V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Levetiracetam SUN
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no frasco e na caixa após CAD:. A data de validade é o último dia do mês que se indica.
Este medicamento não requer condições especiais de conservação.
6. Conteúdo do envase e informação adicional
Composição de Levetiracetam SUN
- O princípio ativo é levetiracetam. Cada ml contém 100 mg de levetiracetam.
- Os outros componentes são: acetato de sódio trihidratado, ácido acético glacial, cloreto de sódio, água para preparações injetáveis.
Aspecto de Levetiracetam SUN e conteúdo do envase
Levetiracetam SUN concentrado para solução para perfusão (concentrado estéril) é um líquido transparente e incolor.
Levetiracetam SUN concentrado para solução para perfusão é acondicionado em caixas de cartão que contêm 10 frascos de 5 ml.
Título da autorização de comercialização e responsável pela fabricação
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Países Baixos
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização.
Bélgica/Bélgica/Bélga/
República Checa/
Dinamarca/Estônia/Grécia/Irlanda/Islândia/
Chipre/Letônia/Lituânia/Luxemburgo/Luxemburgo/Hungria/
Malta/Países Baixos/Noruega/Áustria/Portugal/
República Eslovaca/Finlândia/Finlândia/Suécia
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Países Baixos/Países Baixos/Países Baixos/Нидерланды/Нидерланды/
Países Baixos/Holanda/Ολλανδία/The Netherlands/Holanda/
Ολλανδία/Niederlande/Nyderlandai/Países Baixos/Países Baixos/Holanda/
L-Olanda/Países Baixos/Países Baixos/Países Baixos/Países Baixos/
Holanda/Finlândia/Países Baixos/Países Baixos
Tel./тел./tlf./τηλ./Sími/τηλ./Tlf./Puh./
+31 (0)23 568 5501
Alemanha Sun Pharmaceuticals Germany GmbH Hemmelrather Weg 201 51377 Leverkusen Alemanha Tel. +49 214 403 99 0 Basics GmbH Hemmelrather Weg 201 51377 Leverkusen Alemanha Tel. +49 214 403 99 0 | Polônia Ranbaxy (Polônia) Sp. Z o. o. ul. Idzikowskiego 16 00-71 Varsóvia Polônia tel. +48 22 642 07 75 |
Espanha Sun Pharma Laboratorios .S.L. Rambla de Catalunya 53-55 08007 Barcelona Espanha tel. +34 93 342 78 90 | Romênia Terapia S.A. Str. Fabricii nr 124 Cluj-Napoca, Judetul Cluj Romênia tel. +40 (264) 501 500 |
França Sun Pharma France 31, Rue des Poissonniers 92200 Neuilly-sur-Seine França tel. +33 (0) 1 41 44 44 50 | Eslovênia Lenis farmacevtika d.o.o. Litostrojska cesta 52 1000 Liubliana Eslovênia tel. +386 (0)1 235 07 00 |
Croácia Medicopharmacia d.o.o. Ulica Pere Budmanija 5 10000 Zagreb Croácia tel. +385 1 5584 604 Itália Sun Pharma Italia Srl Viale Giulio Richard, 1 20143 Milão Itália tel. +39 02 33 49 07 93 |
Data da última revisão deste prospecto: Maio/2025
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu
Esta informação está destinada apenas a profissionais do setor sanitário:
As instruções para o uso adequado de Levetiracetam SUN são fornecidas na seção 3.
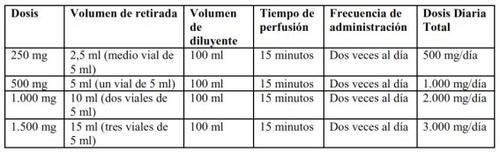
Um frasco de Levetiracetam SUN concentrado contém 500 mg de levetiracetam (5 ml de concentrado de 100 mg/ml). Ver na Tabela 1 a preparação e administração recomendadas de Levetiracetam SUN concentrado para alcançar a dose diária total de 500 mg, 1.000 mg, 2.000 mg ou 3.000 mg repartidos em duas doses.
Tabela 1. Preparação e administração de Levetiracetam SUN concentrado
Este fármaco é de uso único, por isso a solução não utilizada deve ser descartada.
Período de validade em uso: do ponto de vista microbiológico, o produto deve ser utilizado imediatamente após a sua diluição. Em caso de não ser usado imediatamente, o tempo e as condições de armazenamento antes do uso subsequente são responsabilidade do usuário e não devem ser superiores a 24 horas entre 2 e 8°C, a menos que a diluição tenha sido realizada em condições assépticas validadas e controladas.
Verificou-se que Levetiracetam SUN concentrado é fisicamente compatível e quimicamente estável quando misturado com os seguintes diluentes durante pelo menos 24 horas e conservado em bolsas de PVC a temperatura ambiente controlada de 15-25°C.
Diluentes:
- Solução para injeção de cloreto de sódio 9 mg/ml (0,9%)
- Solução para injeção de Ringer lactato
- Solução para injeção de Dextrosa 50 mg/ml (5%)
- País de registo
- Disponibilidade em farmáciasProblema de disponibilidade reportado
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃOForma farmacêutica: PERFURAÇÃO INJETÁVEL, 100 mgSubstância ativa: levetiracetamFabricante: Ucb PharmaRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 100 mg/mlSubstância ativa: levetiracetamFabricante: Ucb PharmaRequer receita médicaForma farmacêutica: SOLUÇÃO/SUSPENSÃO ORAL, 100 mgSubstância ativa: levetiracetamFabricante: Ucb PharmaRequer receita médica
Alternativas a LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO em Polónia
Alternativa a LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO em Ukraine
Médicos online para LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de LEVETIRACETAM SUN 100 MG/ML CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.







