KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO

Pergunte a um médico sobre a prescrição de KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO

Como usar KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO
Introdução
Prospecto: informação para o utilizador
Kivizidiale 40 microgramas/ml + 5 mg/ml colírio em solução
Travoprost/timolol
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe receitado somente a si, e não deve dá-lo a outras pessoas, embora tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem no prospecto. Ver secção 4.
Conteúdo do prospecto:
- O que é Kivizidiale e para que é utilizado
- O que necessita de saber antes de começar a usar Kivizidiale
- Como usar Kivizidiale
- Possíveis efeitos adversos
- Conservação de Kivizidiale
- Conteúdo do envase e informação adicional
1. O que é Kivizidiale e para que é utilizado
Kivizidiale colírio em solução é uma combinação de dois princípios ativos (travoprost e timolol). Travoprost é um análogo de prostaglandinas que actua aumentando o fluxo de saída do fluido aquoso do olho, e por isso diminui a pressão do olho. Timolol é um betabloqueante que actua reduzindo a formação de fluido no interior do olho. As duas substâncias actuem de forma conjunta para reduzir a pressão no interior do olho.
Kivizidiale colírio é utilizado para o tratamento da pressão elevada no olho em adultos, incluindo pacientes de idade avançada. Esta pressão elevada pode causar uma doença denominada glaucoma.
Kivizidiale colírio em solução é uma solução estéril que não contém conservantes.
2. O que necessita de saber antes de começar a usar Kivizidiale
Não use Kivizidiale colírio em solução:
- Se é alérgico a travoprost, prostaglandinas, timolol, betabloqueantes ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
- Se sofre de problemas respiratórios, como asma, bronquite obstructiva crónica grave (doença pulmonar grave que pode causar sibilancias, dificuldade para respirar e/ou tos persistente) ou outros tipos de problemas respiratórios.
- Se sofre de rinite alérgica grave.
- Se apresenta ritmo cardíaco lento, insuficiência cardíaca ou um distúrbio do ritmo cardíaco (latido cardíaco irregular).
- Se a superfície do seu olho está nublada.
Consulte o seu médico se se encontrar em alguma dessas situações.
Advertências e precauções
Consulte o seu médico antes de começar a usar Kivizidiale se tem ou teve no passado:
- Doença coronária (os sintomas podem incluir dor ou opressão no peito, dificuldade para respirar ou sensação de ahogo), insuficiência cardíaca, pressão arterial baixa.
- Alterações da frequência cardíaca, como latido cardíaco lento.
- Problemas respiratórios, asma ou doença pulmonar obstructiva crónica.
- Distúrbios por má circulação sanguínea (como doença de Raynaud ou síndrome de Raynaud).
- Diabetes (já que timolol pode mascarar os sinais e sintomas de níveis baixos de açúcar no sangue).
- Hiperatividade da glândula tireoide (já que timolol pode mascarar os sinais e sintomas de doença da tireoide).
- Miastenia gravis (debilidade muscular crónica).
- Cirurgia de cataratas.
- Inflamação ocular.
Se necessitar de se submeter a algum tipo de operação, informe o seu médico de que está a utilizar Kivizidiale, já que timolol pode modificar os efeitos de alguns medicamentos usados durante a anestesia.
Se sofrer qualquer reação alérgica grave (erupção na pele, enrubescimento e picazón no olho) enquanto utiliza Kivizidiale, qualquer que seja a causa, o tratamento com adrenalina pode não ser tão eficaz. Por isso, é importante que comunique ao médico que está a utilizar Kivizidiale quando for receber qualquer outro tratamento
Kivizidiale pode alterar a cor do íris (parte colorida do olho). Esta alteração pode ser permanente.
Kivizidiale pode aumentar o comprimento, o grosso, a cor e/ou o número das suas pestanas e pode causar um crescimento anormal de pelo nas suas pálpebras.
Travoprost pode ser absorvido pela pele e, por isso, não deve ser utilizado em mulheres grávidas ou que estejam a tentar engravidar. Se este medicamento entrar em contacto com a pele, deve ser lavado de imediato.
Crianças
Kivizidiale não deve ser utilizado em crianças e adolescentes menores de 18 anos de idade.
Outros medicamentos e Kivizidiale
Informa o seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento, incluindo medicamentos que tenha obtido sem receita.
Kivizidiale pode afectar ou ser afectado por outros medicamentos que está a usar, incluindo outros colírios para o tratamento do glaucoma. Informe o seu médico se está a usar ou pretende usar:
- medicamentos para reduzir a pressão arterial,
- medicamentos para o coração, incluindo quinidina (usada para tratar doenças do coração e alguns tipos de malária),
- medicamentos para o tratamento da diabetes ou antidepressivos conhecidos como fluoxetina e paroxetina.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento.
Não utilize Kivizidiale se está grávida, a menos que o seu médico o recomende. Se pode engravidar, deve utilizar um meio de contracepção adequado enquanto utilizar este medicamento.
Não utilize Kivizidiale se está em período de lactação. Este medicamento pode passar para o leite materno.
Condução e uso de máquinas
Kivizidiale pode causar visão borrosa logo após a sua utilização. Não conduza nem use máquinas até que os sintomas tenham desaparecido.
Kivizidiale contém hidroxiestearato de macroglicerol 40
Este medicamento contém hidroxiestearato de macroglicerol 40, que pode produzir reacções cutâneas.
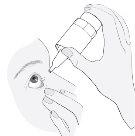
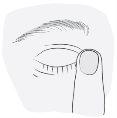
3. Como usar Kivizidiale
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
A dose recomendada é
Uma gota administrada uma vez ao dia, bem de manhã ou bem à noite, no olho ou olhos afectados. Utilize este medicamento todos os dias à mesma hora.
Somente utilize Kivizidiale nos dois olhos se o seu médico o tiver indicado.
Utilize Kivizidiale tanto tempo quanto o seu médico lhe disse.
Kivizidiale só deve ser utilizado como colírio.
Se está a utilizar outros colírios além de Kivizidiale, espere pelo menos 5 minutos entre a aplicação de Kivizidiale e as outras gotas.
Se utiliza lentes de contacto blandas, não utilize as gotas com as lentes colocadas. Espere 15 minutos após lançar as gotas antes de colocar as lentes de contacto de novo.
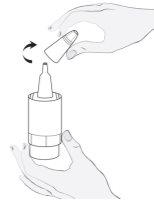
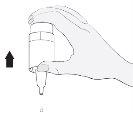
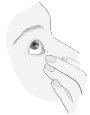
Instruções de uso
1a
1b |
|
2 |
|
3 |
|
4
5 |
|
|
Se usar mais Kivizidiale do que deve
Se usar mais Kivizidiale do que deve, enxágue os olhos com água morna. Não aplique mais gotas até que seja a hora da sua dose habitual.
Em caso de sobredosagem ou ingestão acidental, consulte imediatamente o seu médico ou farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone: 91 562 04 20, indicando o medicamento e a quantidade ingerida.
Se esquecer de usar Kivizidiale
Se esquecer de aplicar Kivizidiale, continue com a próxima dose como estava planeado. Não aplique uma dose dupla para compensar a dose esquecida. A dose não deve exceder 1 gota por dia no (os) olho(s) afectado(s).
Se interromper o tratamento com Kivizidiale
Se deixar de utilizar Kivizidiale sem consultar o seu médico, a pressão no seu olho deixará de estar controlada, o que poderá provocar perda de visão.
Se tiver alguma outra dúvida sobre a utilização deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Em geral, a menos que os efeitos sejam graves, pode continuar a utilizar o colírio. Se tiver alguma dúvida, consulte um médico ou farmacêutico. Não deixe de usar Kivizidiale sem consultar o seu médico.
Efeitos adversos muito frequentes(podem afectar mais de 1 em cada 10 pessoas):
Efeitos no olho
Enrubescimento do olho.
Efeitos adversos frequentes(podem afectar 1 em cada 10 pessoas):
Efeitos no olho
Inflamação da superfície ocular com dano na superfície, dor ocular, visão borrosa, visão anormal, olho seco, picazón no olho, molestias no olho, sinais e sintomas de irritação ocular (p. ex.: queimadura, picazón).
Efeitos adversos pouco frequentes(podem afectar 1 em cada 100 pessoas):
Efeitos no olho
Inflamação da superfície do olho, inflamação da pálpebra, inchação da conjuntiva, aumento do crescimento das pestanas, inflamação do íris, inflamação do olho, sensibilidade à luz, visão reduzida, olhos cansados, alergia ocular, olhos inchados, aumento da produção de lágrimas, enrubescimento da pálpebra, alteração da cor da pálpebra, escurecimento da pele (à volta do olho).
Outros efeitos
Reacção alérgica à substância activa, tontura, dor de cabeça, aumento ou diminuição da pressão sanguínea, falta de ar, crescimento excessivo do pelo, gotejamento na parte posterior da garganta, inflamação e picazón da pele, frequência cardíaca diminuída.
Efeitos adversos raros(podem afectar 1 em cada 1.000 pessoas):
Efeitos no olho
Afinamento da superfície ocular, inflamação das glândulas da pálpebra, rotura de vasos sanguíneos no olho, crostas nas pálpebras, disposição anormal das pestanas, crescimento anormal das pestanas.
Outros efeitos
Nervosismo, frequência cardíaca irregular, perda de cabelo, alterações da voz, dificuldade para respirar, tos, irritação da garganta, urticária, valores anormais nos testes sanguíneos do fígado, decoloração da pele, sede, cansaço, desconforto dentro do nariz, urina colorida, dor nas mãos e pés.
Frequência não conhecida (não pode ser estimada a partir dos dados disponíveis):
Efeitos no olho
Pálpebra caída (provocando que o olho fique meio fechado), olhos fundos (os olhos parecem mais profundos), alterações na cor do íris (parte colorida do olho).
Outros efeitos
Erupção na pele, insuficiência cardíaca, dor no peito, ataque cardíaco, desmaio, depressão, alucinação, asma, aumento do ritmo cardíaco, sensação de formigueiro ou entorpecimento, palpitações, inchação nos membros inferiores, mau sabor de boca.
Assim como:
Kivizidiale é uma associação de 2 substâncias activas, travoprost e timolol. Como outros medicamentos administrados nos olhos, travoprost e timolol (um betabloqueante) são absorvidos e passam para o sangue. Isso pode causar efeitos adversos semelhantes aos observados com os medicamentos betabloqueantes administrados por via oral ou por injeção. A incidência de efeitos adversos após a administração nos olhos é inferior à quando os medicamentos são administrados por via oral ou injetados.
Os efeitos adversos enumerados a seguir incluem reacções observadas com a classe de betabloqueantes utilizados para tratar doenças do olho, ou reacções observadas com travoprost só:
Efeitos no olho
Inflamação da pálpebra, inflamação na córnea, desprendimento da camada situada por debaixo da retina que contém os vasos sanguíneos que podem causar alterações da visão após uma cirurgia de filtração, sensibilidade corneal diminuída, erosão corneal (dano na face anterior do globo ocular), visão dupla, secreção do olho, inchação à volta do olho, picazón na pálpebra, volta anormal para fora da pálpebra com enrubescimento, irritação e aumento na produção de lágrimas, visão borrosa (sinal de opacidade do cristalino), inchação de uma parte do olho (úvea), eczema das pálpebras, visão de halos, sensibilidade diminuída no olho, pigmentação dentro do olho, pupilas dilatadas, alteração da cor das pestanas, alteração da textura das pestanas, campo visual anormal.
Outros efeitos
- Distúrbios do ouvido e do labirinto: tonturas com sensação de vertigem, zumbidos nos ouvidos.
- Coração e circulação: frequência cardíaca lenta, palpitações, edema (acumulação de líquido), alterações no ritmo ou na frequência do latido cardíaco, insuficiência cardíaca congestiva (doença do coração com dificuldade para respirar e inchação dos pés e das pernas devido à acumulação de líquidos), um tipo de distúrbio do ritmo cardíaco, ataque cardíaco, diminuição da tensão arterial, fenómeno de Raynaud, mãos e pés frios, redução do fluxo sanguíneo para o cérebro.
- Respiratório: constrição das vias respiratórias dos pulmões (principalmente em pacientes com doença pré-existente), nariz que moqueia ou entupido, espirros (devido à alergia), dificuldade para respirar, sangramentos de nariz, secura nasal.
- Distúrbios gerais e do sistema nervoso: dificuldade para dormir (insónia), pesadelos, perda de memória, perda de força e energia, ansiedade (angústia emocional excessiva).
- Distúrbios gastrointestinais: alteração do gosto, náuseas, indigestão, diarreia, boca seca, dor abdominal, vómitos, constipação.
- Alergia: aumento dos sintomas alérgicos, reacções alérgicas generalizadas, incluindo inchação por baixo da pele que pode produzir-se em áreas como a face e as extremidades, e podem obstruir as vias aéreas e causar dificuldade para engolir ou respirar, erupção localizada e generalizada, picazón, reacção alérgica repentina grave que pode comprometer a vida.
- Pele: erupção cutânea com aspecto branco prateado (erupção psoriasiforme) ou piora da psoríase, descamação da pele, textura anormal do cabelo, inflamação da pele com sarpullido e enrubescimento, alteração da cor do cabelo, perda de pestanas, picazón, crescimento anormal do cabelo, enrubescimento da pele.
- Muscular: aumento dos sinais e sintomas da miastenia gravis (distúrbio muscular), sensação incomum como de agulhas ou picadas, fraqueza muscular/cansaço, dor muscular não causada pelo exercício, dor nas articulações.
- Distúrbios renais e urinários: dificuldade e dor ao urinar, perda de urina involuntária.
- Reprodução: disfunção sexual, diminuição da libido.
- Metabolismo: níveis baixos de açúcar no sangue, aumento do marcador do cancro da próstata.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informação sobre a segurança deste medicamento.
5. Conservação de Kivizidiale
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na etiqueta do frasco e na caixa após «CAD». A data de validade é o último dia do mês que se indica.
Não utilize este medicamento se observar que o lacre de segurança do frasco está quebrado antes de utilizá-lo pela primeira vez.
Não conserve a uma temperatura superior a 25ºC.
Para evitar infecções, deve descartar o frasco do medicamento 28 dias após tê-lo aberto pela primeira veze utilizar um frasco novo. Escreva a data de abertura do frasco na etiqueta do frasco e na caixa no espaço designado para isso.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os recipientes e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Kivizidiale
- Os princípios ativos são travoprost e timolol. Cada ml de solução contém 40 microgramas de travoprost e 5 mg de timolol (como timolol).
- Os demais componentes são manitol (E421), ácido bórico, hidróxido de sódio (para o ajuste do pH), hidroxiestearato de macroglicerol (valor nominal: 40), propilenglicol (E1520), cloreto de sódio e água purificada.
Aspecto do produto Kivizidiale e conteúdo do envase
Kivizidiale colírio em solução apresenta-se como uma solução aquosa de 2,5 ml, transparente, incolor e praticamente livre de partículas, em um recipiente multidose branco (PP) de 5 ml com um sistema de bombeamento (PP, HDPE, LDPE) e um cilindro de pressão e tampa (HDPE) contido em uma caixa de cartão.
O produto está disponível nos seguintes tamanhos de envase:
Caixas que contêm 1 ou 3 frascos.
Pode ser que nem todos os tamanhos de envase estejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
Titular da Autorização de Comercialização:
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Irlanda
Responsável pela Fabricação:
Pharmathen SA,
6 Dervenakion Str,
153 51 Pallini
Grécia
Ou
JADRAN - GALENSKI LABORATORIJ d.d.
Svilno 20,
51000 Rijeka
Croácia
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do Titular da Autorização de Comercialização.
Representante Local em Portugal
Bausch & Lomb S.A.
Avda. Valdelaparra, 4
28108 – Alcobendas, Madrid
Tel: 91 – 657 63 00
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
AT Kivizidiale 40 Mikrogramm/ml + 5 mg/ml Augentropfen Lösung
BE Kivizidiale 40 microgram/ml + 5 mg/ml oogdruppels, oplossing
BG ?????????? 40 ??????????/ml + 5 mg/ml ????? ?? ???, ???????
CY Kivizidiale
DE Kivizidiale 40 Mikrogramm/ml + 5 mg/ml Augentropfen, Lösung
DK Kivizidiale
EE Kivizidiale
ES Kivizidiale 40 μg/ml + 5 mg/ml colirio en solución
FR Kivizidiale 40 microgrammes/mL + 5 mg/mL, collyre en solution
EL Kivizidiale
HR Kivizidiale 40 mikrograma/ml + 5 mg/ml, kapi za oko, otopina
HU Kivizidiale 40 mikrogramm/ml + 5 mg/ml oldatos szemcsepp
NL Kivizidiale 40 microgram/ml + 5 mg/ml oogdruppels, oplossing
LT Kivizidiale 40 mikrogramu/ 5 mg/ ml akiu lašai (tirpalas)
LU Kivizidiale 40 microgrammes/ml + 5 mg/ml collyre en solution
PL Kivizidiale
PT Kivizidiale 40 μg/ml + 5 mg/ml colírio, solução
RO Kivizidiale 40 micrograme/mL + 5 mg/mL picaturi oftalmice, solu?ie
SK Kivizidiale 40 mikrogramov/ml + 5 mg/ml
Data da última revisão deste prospecto: março 2022.
A informação detalhada deste medicamento está disponível na página web da Agência Portuguesa de Medicamentos e Produtos de Saúde (INFARMED) http://www.infarmed.pt/.

Quanto custa o KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO em Espanha em 2025?
O preço médio do KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO em dezembro de 2025 é de cerca de 7.84 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia7.84 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃOForma farmacêutica: COLÍRIO, 10 mg/ml + 5 mg/mlSubstância ativa: timolol, combinationsFabricante: Novartis Europharm LimitedRequer receita médicaForma farmacêutica: COLÍRIO, 0,3 mg/ml + 5 mg/mlSubstância ativa: timolol, combinationsFabricante: Brill Pharma S.L.Requer receita médicaForma farmacêutica: COLÍRIO, 0,3 mg/ml + 5 mg/mlSubstância ativa: timolol, combinationsFabricante: Laboratorio Stada S.L.Requer receita médica
Alternativas a KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO em Polónia
Alternativa a KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO em Ukraine
Médicos online para KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de KIVIZIDIALE 40 microgramas/ml + 5 mg/ml COLÍRIO EM SOLUÇÃO – sujeita a avaliação médica e regras locais.