GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO

Pergunte a um médico sobre a prescrição de GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO

Como usar GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO
Introdução
Prospecto:informação para o paciente
Gadovist 1 mmol/ml solução injetável em seringa precarregada/
cartucho precarregado
Gadobutrol
Leia todo o prospecto atentamente antes de começar a usar este medicamento,porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou a pessoa que lhe administre Gadovist (o radiologista) ou o pessoal do hospital ou centro onde se realizar a RM (ressonância magnética).
- Se experimentar efeitos adversos, consulte o seu médico ou radiologista, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Gadovist e para que se utiliza
- O que precisa saber antes de começar a usar Gadovist
- Como usar Gadovist
- Possíveis efeitos adversos
- Conservação de Gadovist
- Conteúdo do envase e informação adicional
1. O que é Gadovist e para que se utiliza
Gadovist é um meio de contraste para ressonância magnética (RM) usado para o diagnóstico do cérebro, coluna vertebral e vasos sanguíneos. Gadovist também pode ajudar o médico a averiguar o tipo de anormalidades (benignas ou malignas) conhecidas ou suspeitas no fígado e rins.
Gadovist também pode ser utilizado para RM de anormalidades de outras partes do corpo. Facilita a visualização de estruturas anormais ou lesões e ajuda na diferenciação do tecido saudável e do tecido doente.
Está indicado em adultos e crianças de todas as idades (incluindo neonatos a termo).
Como funciona Gadovist
A RM é um método de diagnóstico por imagem que utiliza o comportamento das moléculas de água em tecidos normais e anormais. Isso é realizado mediante um complexo sistema de ímãs e ondas de rádio. Computadores registram a atividade e a transformam em imagens.
Gadovist é administrado mediante uma injeção na sua veia. Este medicamento é único para uso diagnóstico e só lhe será administrado por um profissional de saúde com experiência na prática clínica de RM.
2. O que precisa saber antes de começar a usar Gadovist
Não use Gadovist se você
- é alérgico ao gadobutrol ou a algum dos outros componentes deste medicamento (incluídos na seção 6).
Advertências e precauções
Consulte o seu médico antes de começar a usar Gadovist se você
- sofre ou sofreu de uma alergia (por exemplo, febre do feno, urticária) ou asma
- teve uma reação prévia a qualquer meio de contraste
- tem a função renal muito deficiente
- sofre de distúrbios cerebrais com convulsões (ataques) ou tem outras doenças do sistema nervoso
- usa um marcapasso ou algum implante ou clipe que contenha ferro no seu corpo.
Seu médico decidirá se é possível ou não realizar a exploração prevista.
- Podem ocorrer reações de tipo alérgico ou outros tipos de reações que envolvam problemas cardíacos, dificuldade ao respirar ou reações cutâneas após o uso de Gadovist. É possível que apareçam reações graves. A maioria dessas reações ocorre na meia hora após a administração de Gadovist. Por isso, você será observado após o tratamento. Foram observadas reações retardadas (após horas ou dias) (ver seção 4).
Rins/Fígado
Informa ao seu médico se
- seus rins não funcionam corretamente
- se recentemente se realizou, ou em breve se realizará, um transplante de fígado.
Seu médico pode decidir realizar um exame de sangue para comprovar o funcionamento correto dos seus rins antes de decidir o uso de Gadovist, especialmente se você tem 65 anos ou mais.
Neonatose lactentes
Devido à imaturidade da função renal dos neonatos de até 4 semanas e lactentes de até 1 ano de idade, Gadovist só deve ser utilizado nesses pacientes após uma avaliação cuidadosa pelo médico.
Outros medicamentos e Gadovist
Informa ao seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento.
Gravidez e lactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
- Gravidez
Gadobutrol pode atravessar a placenta. Desconhece-se se afeta o feto. Deve informar ao seu médico se acredita que está grávida ou que possa estar, pois Gadovist não deve ser utilizado durante a gravidez a menos que se considere absolutamente necessário.
- Lactação
Informa ao seu médico se está amamentando ou está prestes a começar. Seu médico avaliará se você deve continuar ou se deve interromper a lactação 24 horas após a administração de Gadovist.
Gadovist contém sódio
Este medicamento contém menos de 23 mg de sódio por dose (com base na quantidade média administrada a uma pessoa de 70 kg); isto é, essencialmente "isento de sódio".
3. Como usar Gadovist
Gadovist é injetado por um profissional de saúde mediante uma pequena agulha dentro de uma veia. Sua exploração de RM pode começar imediatamente.
Após a injeção, permanecerá em observação durante pelo menos 30 minutos.
Posologia habitual
A dose habitual que é adequada para você dependerá do seu peso corporal e da região examinada por RM:
Em adultos, recomenda-se uma única injeção de 0,1 mililitros de Gadovist por kg de peso corporal (isso significa que para uma pessoa que pesa 70 kg a dose seria de 7 mililitros), no entanto, pode ser administrada uma injeção adicional de até 0,2 mililitros por kg de peso corporal dentro de 30 minutos após a primeira injeção. Pode ser administrada uma quantidade total de 0,3 mililitros de Gadovist por kg de peso corporal como máximo (isso significa que para uma pessoa de 70 kg a dose seria de 21 mililitros) para a obtenção de imagens do sistema nervoso central (SNC) e da angiografia por ressonância magnética (ARM) com contraste. Pode ser administrada uma dose mínima de 0,075 mililitros de Gadovist por kg de peso corporal (isso significa que para uma pessoa de 70 kg a dose seria de 5,25 mililitros) para o SNC.
No final do prospecto, inclui-se informação adicional sobre a administração e manipulação de Gadovist.
Posologia em populações especiais
O uso de Gadovist não é recomendado em pacientes com problemas renais graves ou em pacientes a quem recentemente se realizou, ou em breve se realizará, um transplante de fígado. No entanto, se for necessário o uso, durante uma exploração, só deve ser administrada uma dose de Gadovist e não deve ser administrada uma segunda injeção até que tenham transcorrido pelo menos 7 dias.
Uso emneonatos, lactentes,crianças e adolescentes
A dose recomendada em crianças de todas as idades (incluindo neonatos a termo) é de uma única injeção de 0,1 mililitros de Gadovist por quilograma de peso corporal para todas as indicações (ver seção 1).
Devido à imaturidade da função renal dos neonatos de até 4 semanas e lactentes de até 1 ano de idade, Gadovist só deve ser utilizado nesses pacientes após uma avaliação cuidadosa pelo médico. Os neonatos e lactentes só devem receber uma dose de Gadovist durante uma exploração e não devem receber uma segunda injeção até transcorridos pelo menos 7 dias.
Pacientes de idade avançada
Se você tem 65 anos ou mais, não é necessário que a dose seja ajustada, mas pode ser realizado um exame de sangue para comprovar o funcionamento correto dos seus rins.
Se usa mais Gadovist do que deve
É improvável que ocorra uma sobredose. Se ocorrer, o médico tratará todos os sintomas e pode utilizar diálise para eliminar Gadovist do seu corpo. Não há evidência que indique que a hemodiálise é adequada para a prevenção do desenvolvimento de fibrose nefrogênica sistêmica (FNS; ver seção 4) por isso não deve ser utilizada para o tratamento desta doença. Em alguns casos, o seu coração será controlado.
Em caso de sobredose ou ingestão acidental, consulte o Serviço de Informação Toxicológica (telefone 91 562 04 20).
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou radiologista.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram. A maioria dessas reações ocorre na meia hora seguinte à administração de Gadovist. Foram observadas, em casos raros, reações de tipo alérgico retardadas ou de outro tipo, desde algumas horas a vários dias após ter recebido Gadovist. Se isso lhe acontecer, informe imediatamente ao seu médico ou radiologista.
Os efeitos adversos mais graves(que foram fatais ou que colocaram em perigo a vida em alguns casos) são:
- parada do batimento do coração (parada cardíaca) uma doença pulmonar grave (síndrome de dificuldade respiratória aguda)/ líquido nos pulmões (edema pulmonar)e reações alérgicas graves (anafilactoides) (incluindo parada respiratória e choque).
Adicionalmente, em alguns casos foram observados os seguintes efeitos adversos que colocaram em perigo a vida ou foram fatais:
- falta de respiração (dispnéia), perda de consciência, reações alérgicas graves, diminuição grave da tensão que pode conduzir a colapso, parada respiratória, líquido nos pulmões, inflamação da boca e garganta e tensão baixa.
Em casos raros:
- podem ocorrer reações de tipo alérgico(hipersensibilidade e anafilaxia), incluindo reações graves (choque) que podem requerer intervenção médica imediata.
Se você nota:
- inflamação do rosto, lábios, língua ou garganta
- tosse e espirros
- dificuldade para respirar
- coceira
- congestão nasal
- urticária (erupção cutânea como a causada pela ortiga)
informe imediatamente ao pessoal do departamento de RM.Estes podem ser os primeiros indícios de que está ocorrendo uma reação grave. Sua exploração pode ser suspensa e você pode precisar de tratamento posterior.
Os efeitos adversos observados com maior frequência(podem afetar 5 ou mais de cada 1.000 pessoas) são:
- cefaleias, sensação de mal-estar (náuseas) e tontura.
A maioria dos efeitos adversos é de leves a moderados.
A seguir, são enumerados possíveis efeitos adversosque foram observados em estudos clínicosantes da aprovação de Gadovist, de acordo com sua probabilidade:
Frequentes:podem afetar até 1 de cada 10 pessoas
- dor de cabeça
- sensação de mal-estar (náuseas)
Pouco frequentes:podem afetar até 1 de cada 100 pessoas
- reação de tipo alérgico, por exemplo:
- tensão baixa
- urticária
- inflamação do rosto
- inflamação (edema) das pálpebras
- rubor
A frequência das seguintes reações alérgicas é desconhecida:
- uma reação alérgica grave (choque anafilactoide)
- diminuição grave da tensão que pode conduzir a colapso (choque)
- parada respiratória
- dificuldade ao respirar (broncoespasmo)
- lábios azuis
- inflamação da boca e da garganta
- inflamação da garganta
- aumento da tensão
- dor no peito
- inflamação do rosto, garganta, boca, lábios e/ou língua (angioedema)
- conjuntivite
- aumento da sudorese
- tosse
- espirros
- queimadura
- palidez (pele pálida)
- tontura, alteração do gosto, entorpecimento e formigamento
- falta de respiração (dispnéia)
- vômito
- vermelhidão na pele (eritema)
- coceira (prurido incluído prurido generalizado)
- erupção (incluindo erupção generalizada, pequenas manchas vermelhas planas (erupção macular), lesões pequenas elevadas circunscritas (erupção papular), erupção com coceira (erupção prurítica))
- diferentes tipos de reações no local de injeção (por exemplo, derrame no tecido adjacente, queimadura, frio, calor, vermelhidão, erupção, dor ou hematoma)
- sensação de calor
Raros:podem afetar até 1 de cada 1.000 pessoas
- desmaio
- convulsão
- alteração do olfato
- batimento rápido do coração
- palpitações
- secura da boca
- mal-estar geral
- sensação de frio
Efeitos adversos adicionais que foram comunicados após a aprovação de Gadovist de frequência desconhecida(a frequência não pode ser estimada a partir dos dados disponíveis):
- parada do batimento do coração (parada cardíaca)
- Uma doença pulmonar grave (síndrome de dificuldade respiratória aguda)
- Líquido nos pulmões (edema pulmonar)
- foram notificados casos de fibrose nefrogênica sistêmica – FNS (que provoca um endurecimento da pele e pode afetar também os tecidos moles e os órgãos internos).
Após a administração de Gadovist, foram observadas variações nos resultados de análise da função renal (por exemplo, aumentos da creatinina no soro).
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou radiologista, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Gadovist
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na etiqueta e no envase após CAD. A data de validade é o último dia do mês que se indica. Este medicamento não requer condições especiais de conservação.
Foi demonstrada a estabilidade química, física e microbiológica no uso durante um prazo de 24 horas a 20-25ºC. Desde o ponto de vista microbiológico, o produto deve ser utilizado imediatamente após a abertura.
Este medicamento é uma solução clara, de incolora a amarelo pálido. Não utilize este medicamento se observar uma alteração severa da cor, ou a presença de partículas ou o envase aparece defeituoso.
Os medicamentos não devem ser jogados nos desagües nem na lixeira. O profissional de saúde se desfará deste medicamento quando não for necessário. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Gadovist
O princípio ativo é o gadobutrol.
1 ml de solução injetável contém 604,72 mg de gadobutrol (equivalente a 1 mmol de gadobutrol que contém 157,25 mg de gadolínio).
1 seringa pré-carregada com 5,0 ml contém 3.023,6 mg de gadobutrol.
1 seringa pré-carregada com 7,5 ml contém 4.535,4 mg de gadobutrol.
1 seringa pré-carregada com 10 ml contém 6.047,2 mg de gadobutrol.
1 seringa pré-carregada com 15 ml contém 9.070,8 mg de gadobutrol.
1 seringa pré-carregada com 20 ml contém 12.094,4 mg de gadobutrol.
1 cartucho pré-carregado com 15 ml contém 9.070,8 mg de gadobutrol.
1 cartucho pré-carregado com 20 ml contém 12.094,4 mg de gadobutrol.
1 cartucho pré-carregado com 30 ml contém 18.141,6 mg de gadobutrol.
Os outros componentes são calcobutrol de sódio (ver seção 2), trometamol, ácido clorídrico 1N e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Gadovist é uma solução injetável transparente, de incolora a amarelo pálido.
O conteúdo dos envases é:
-1 ou 5 seringas pré-carregadas que contêm 5, 7,5 e 10 ml de solução injetável (em uma seringa de 10 ml de vidro ou plástico).
-1 ou 5 seringas pré-carregadas que contêm 15 ml de solução injetável (em uma seringa de 17 ml de vidro ou em uma seringa de 20 ml de plástico).
-1 ou 5 seringas pré-carregadas que contêm 20 ml de solução injetável (em uma seringa de 20 ml de vidro ou plástico).
-1 ou 5 cartuchos pré-carregados que contêm 15, 20 e 30 ml de solução injetável (em um cartucho de 65 ml).
Envases clínicos:
- 5 seringas pré-carregadas que contêm 5, 7,5, 10, 15, 20 ml de solução injetável
- 5 cartuchos pré-carregados que contêm 15, 20, 30 ml de solução injetável
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização
Bayer Hispania, S.L.
Av. Baix Llobregat, 3-5
08970 Sant Joan Despí (Barcelona)
Espanha
Responsável pela fabricação
Bayer AG
Müllerstrasse 178
13353 Berlim
Alemanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Áustria, Alemanha | Gadovist 1,0 mmol/ml Injektionslösung in Fertigspritzen/Patronen |
Bélgica, Bulgária, Chipre, Dinamarca, Estónia, Finlândia, Grécia, Itália, Luxemburgo, Noruega, Portugal, Suécia | Gadovist |
Croácia | Gadovist 1,0 mmol/ml otopina za injekciju u napunjenoj štrcaljki/ulošku. |
França | GADOVIST 1,0 mmol/ml, solution injectable en seringue préremplie |
Islândia | Gadovist 1,0 mmól/ml stungulyf, lausn í áfylltum sprautum/rörlykjum |
Irlanda | Gadovist 1.0 mmol/ml solution for Injection in prefilled syringe Gadovist 1.0 mmol/ml solution for Injection in prefilled cartridge |
Holanda | Gadovist 1,0 mmol/ml, oplossing voor injectie in voorgevulde spuit/patroon |
Eslovênia | Gadovist 1,0 mmol/ml raztopina za injiciranje v napolnjeni injekcijski brizgi/vložku |
Eslováquia | Gadovist 1,0 mmol/ ml |
Espanha | Gadovist 1 mmol/ml solução injetável em seringa pré-carregada/ cartucho pré-carregado |
Malta | Gadovist 1.0 mmol/ml solution for injection pre-filled syringe/cartridge |
Data da última revisão deste prospecto:09/2024.
Outras fontes de informação
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es)
-----------------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais do setor sanitário:
- Insuficiência renal
Antes da administração de Gadovist se recomenda avaliar a todos os pacientes para detectar uma possível disfunção renal mediante provas de laboratório.
Foram notificados casos de fibrose nefrogênica sistémica (FNS) associados à utilização de alguns agentes de contraste de gadolínio em pacientes com insuficiência renal grave aguda ou crónica (TFG ou taxa de filtração glomerular < 30ml/minuto/1,73m2). Os pacientes submetidos a transplante hepático têm um risco especial, pois a incidência de um falha renal é elevada neste grupo. Posto que existe a possibilidade de que possa ocorrer uma FNS com Gadovist, este só deve ser utilizado em pacientes com insuficiência renal grave e em pacientes no período perioperatório de um transplante hepático após uma avaliação cuidadosa do risco/benefício e se a informação diagnóstica é imprescindível e não pode estar disponível mediante ressonância magnética sem contraste. Se for necessário o uso de Gadovist, a dose não deve exceder 0,1 mmol/kg de peso corporal. Durante uma exploração não deve ser administrada mais de uma dose. Devido à ausência de informação sobre a administração repetida, a administração de Gadovist não deve ser repetida a não ser que tenha transcorrido um intervalo entre injeções de pelo menos 7 dias.
Desde que a eliminação renal de Gadovist pode estar reduzida nos pacientes de idade avançada, é especialmente importante avaliar os pacientes de 65 anos e mais para detectar uma possível disfunção renal.
A hemodiálise pouco após a administração de Gadovist pode resultar útil para a eliminação corporal de Gadovist. Não há evidência que apóie o início da hemodiálise para a prevenção ou tratamento da FNS em pacientes que ainda não estão submetidos a hemodiálise.
- Gravidez e Lactação
Não deve ser utilizado Gadovist durante a gravidez a não ser que a situação clínica da mulher requeira tratamento com Gadovist.
A continuação ou a interrupção da lactação 24 horas após a administração de Gadovist, ficará a critério do médico e da mãe em período de lactação.
- Reações de hipersensibilidade
Como ocorre com outros meios de contraste intravenosos, Gadovist pode associar-se a reações de hipersensibilidade/anafilactoides ou a outras reações idiossincrásicas caracterizadas por manifestações cardiovasculares, respiratórias ou cutâneas, que abrangem até reações graves incluyendo choque. Em geral, pacientes com doenças cardiovasculares são mais suscetíveis a consequências graves ou mesmo fatais por reações de hipersensibilidade graves.
O risco de reações de hipersensibilidade pode ser maior nos seguintes casos:
- reação prévia a meios de contraste
- antecedentes de asma brônquica
- antecedentes de distúrbios alérgicos
Em pacientes com predisposição alérgica, a decisão de utilizar Gadovist deve ser realizada após uma avaliação cuidadosa da relação benefício-riesgo.
A maioria dessas reações ocorre à meia hora após a administração. Por isso, se recomenda a observação do paciente após o tratamento.
É necessário dispor da medicação adequada para o tratamento das reações de hipersensibilidade, bem como preparar a aplicação de medidas de emergência.
Em raros casos, foram observadas reações retardadas (após horas ou vários dias).
- Distúrbios convulsivos
Assim como com outros meios de contraste que contêm gadolínio, deve-se tomar especial precaução nos pacientes com um limiar convulsivo baixo.
- Sobredosagem
Em caso de uma sobredosagem involuntária, se recomenda a monitorização cardiovascular (incluindo o ECG) e a vigilância da função renal como medidas de precaução.
Em caso de sobredosagem em pacientes com insuficiência renal, Gadovist pode ser eliminado mediante hemodiálise. Após 3 sessões de hemodiálise, se elimina do corpo aproximadamente 98% do agente de contraste. No entanto, não há evidência de que a hemodiálise seja adequada para a prevenção de fibrose nefrogênica sistémica (FNS).
- Antes da injeção
Este medicamento está indicado para um único uso.
Este medicamento é uma solução transparente, de incolora a amarelo pálido. A solução deve ser inspecionada visualmente antes de ser utilizada.
Gadovist não deve ser utilizado em caso de apresentar alterações significativas do cor, de aparecimento de partículas ou em caso de que o envase esteja defeituoso.
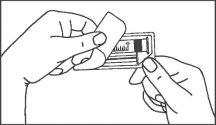
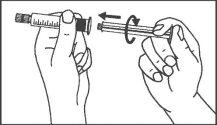
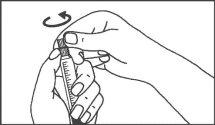
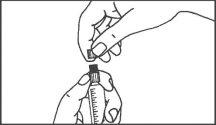
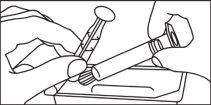
- Instruções de uso
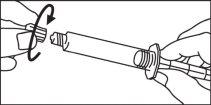
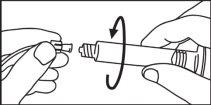
Seringas pré-carregadas
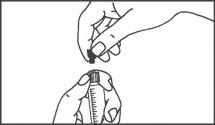
A seringa pré-carregada deve ser extraída do envase e preparada para a injeção imediatamente antes da administração.
O extremo do tapão deve ser retirado da seringa pré-carregada imediatamente antes do uso.
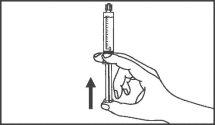
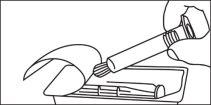
Seringa de vidro:
INJEÇÃO MANUAL | ||
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
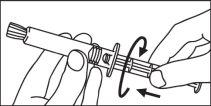
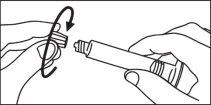
Seringa de plástico:
INJEÇÃO MANUAL | INJEÇÃO COM INJETOR | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
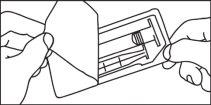
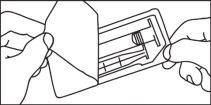
Cartuchos pré-carregados
A administração de meios de contraste deve ser realizada por pessoal qualificado mediante os procedimentos e equipamentos adequados.
A administração de meios de contraste deve ser realizada mediante uma técnica estéril.
O meio de contraste deve ser administrado mediante um injetor do tipo MEDRAD Spectris®.
Devem ser seguidas as instruções fornecidas pelos fabricantes dos dispositivos utilizados.
Qualquer meio de contraste não utilizado em uma exploração deve ser descartado de acordo com as normas locais.
Período de validade após a primeira abertura do envase
Qualquer solução para injeção que não tenha sido utilizada em uma exploração deve ser descartada. Foi demonstrada a estabilidade química, física e microbiológica no uso durante 24 horas a 20-25ºC. Desde um ponto de vista microbiológico, o produto deve ser utilizado imediatamente. Se não for utilizado de maneira imediata, os tempos de armazenamento durante o uso e as condições prévias à utilização são responsabilidade do usuário.
A etiqueta separável das seringas pré-carregadas/cartuchos pré-carregados deve ser colada na história do paciente a fim de permitir um registro preciso do meio de contraste de gadolínio utilizado. Também deve ser registrada a dose utilizada. No caso de se utilizarem registros eletrónicos de pacientes, devem ser introduzidos nos mesmos o nome do produto, o número de lote e a dose administrada.
Posologia
Deve ser utilizada a dose mais baixa que proporcione realce suficiente para fins diagnósticos. A dose deve ser calculada em função do peso corporal do paciente e não deve superar a dose recomendada por quilograma de peso corporal indicada nesta seção.
- Adultos
Indicações no SNC
A dose recomendada em adultos é de 0,1 mmol por quilograma de peso corporal (mmol/kg p.c.), equivalente a 0,1 ml/kg p.c. da solução 1,0 M.
Se persiste uma suspeita clínica fundada da existência de uma lesão apesar de uma RM sem achados patológicos ou quando a obtenção de uma informação mais precisa pode influir sobre o tratamento do paciente, pode ser administrada uma dose adicional de até 0,2 ml/kg p.c. durante os 30 minutos seguintes à primeira injeção. Pode ser administrada uma dose de 0,075 mmol de gadobutrol por kg de peso corporal (equivalente a 0,075 ml de Gadovist por kg de peso corporal) como mínimo para a obtenção de imagens do SNC.
RM de corpo inteiro (excepto ARM)
Em geral, a administração de 0,1 ml de Gadovist por kg de peso corporal é suficiente para responder à pergunta clínica.
Angiografia por Ressonância Magnética (ARM) com contraste
Obtenção de imagens de 1 campo de visão (FOV): 7,5 ml para pesos corporais inferiores a 75 kg; 10 ml para pesos corporais iguais ou superiores a 75 kg (equivalente a 0,1-0,15 mmol/kg p.c.).
Obtenção de imagens de mais de 1 campo de visão (FOV): 15 ml para pesos corporais inferiores a 75 kg; 20 ml para pesos corporais iguais ou superiores a 75 kg (equivalente a 0,2-0,3 mmol/kg p.c.).
- População pediátrica
A dose recomendada em crianças de todas as idades (incluindo neonatos a termo) é de 0,1 mmol de gadobutrol por quilograma de peso corporal (equivalente a 0,1 ml de Gadovist por quilograma de peso corporal) para todas as indicações (ver seção 1).
Devido à imaturidade da função renal dos neonatos de até 4 semanas e lactentes de até 1 ano de idade, Gadovist só deve ser utilizado nestes pacientes após uma avaliação cuidadosa a uma dose não superior a 0,1 mmol/kg de peso corporal. Durante uma exploração não deve ser administrada mais de uma dose. Devido à ausência de informação sobre a administração repetida, a administração de Gadovist não deve ser repetida a não ser que tenha transcorrido um intervalo entre injeções de pelo menos 7 dias.
Imagens
A dose requerida é administrada por via intravenosa como injeção em bolo. A RM com contraste pode começar imediatamente após (pouco após a injeção, dependendo das sequências de pulsos empregadas e do protocolo de estudo).
É observado um realce óptimo da sinal durante o primeiro passo arterial para a ARM com contraste e durante um período de aproximadamente 15 minutos após a injeção de Gadovist para as indicações do sistema nervoso central (SNC) (o momento depende do tipo de lesão/tecido).
As sequências de imagem ponderadas em T1 são especialmente adequadas para as explorações com contraste.
É fornecida informação adicional sobre a utilização de Gadovist na seção 3 do prospecto.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDOForma farmacêutica: INJETÁVEL, 604.72 mg gadobutrol/mlSubstância ativa: gadobutrolFabricante: Bayer Hispania S.L.Requer receita médicaForma farmacêutica: INJETÁVEL, 1,0 mmol/mlSubstância ativa: gadobutrolFabricante: Ge Healthcare Bio-Sciences, S.A.U.Requer receita médicaForma farmacêutica: INJETÁVEL, 1,0 mmol/mlSubstância ativa: gadobutrolFabricante: Ge Healthcare Bio-Sciences, S.A.U.Requer receita médica
Alternativas a GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO em Polónia
Alternativa a GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO em Ukraine
Médicos online para GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de GADOVIST 1 mmol/ml SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA/CARTUCHO PREENCHIDO – sujeita a avaliação médica e regras locais.