EYLEA 40 mg/ml Solução injetável em seringa pré-carregada

Pergunte a um médico sobre a prescrição de EYLEA 40 mg/ml Solução injetável em seringa pré-carregada

Como usar EYLEA 40 mg/ml Solução injetável em seringa pré-carregada
Introdução
Prospecto: informação para o paciente adulto
Eylea 40 mg/ml solução injetável em seringa pré-carregada
aflibercepte
ADULTOS
Se deseja informação para os tutores de bebês nascidos prematuramente, consulte ao final da seção 6”
Leia todo o prospecto atentamente antes de que lhe administrem este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico.
- Se experimentar efeitos adversos, consulte o seu médico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Eylea e para que é utilizado
- O que necessita saber antes de que lhe administrem Eylea
- Como lhe será administrado Eylea
- Possíveis efeitos adversos
- Conservação de Eylea
- Conteúdo do envase e informação adicional
1. O que é Eylea e para que é utilizado
Eylea é uma solução que se injeta no olho para tratar algumas doenças oculares em pacientes adultos, denominadas:
- degeneração macular associada à idade neovascular (exsudativa) comumente conhecida como DMAE exsudativa
- alteração da visão devida ao edema macular causado por um bloqueio das veias retinianas (oclusão da veia central da retina (OVCR) ou da ramo venosa da retina (ORVR))
- alteração da visão devida ao edema macular diabético (EMD)
- alteração da visão devida à neovascularização coroide miópica (NVC miópica).
Aflibercepte, o princípio ativo de Eylea, bloqueia a atividade de um grupo de fatores denominados fator de crescimento endotelial vascular A (VEGF-A) e fator de crescimento placentário (PlGF).
Em pacientes com DMAE exsudativa e NVC miópica, quando estes fatores existem em quantidade excessiva influem na formação anômala de novos vasos sanguíneos no olho. Estes novos vasos sanguíneos podem causar uma fuga dos componentes do sangue para o interior do olho, com o consequente dano nos tecidos oculares responsáveis pela visão.
Em pacientes com OVCR, produz-se um bloqueio da veia principal que transporta sangue desde a retina. Devido a isso, os níveis de VEGF aumentam causando a fuga de fluido na retina e, portanto, a hinchazón da mácula (a parte da retina responsável pela visão fina), o que se conhece como edema macular.
Quando a mácula se enche de líquido, a visão central se torna borrosa.
Em pacientes com ORVR, produz-se um bloqueio de uma ou mais ramas do vaso sanguíneo principal que transporta sangue desde a retina. Devido a isso, os níveis de VEGF aumentam causando a fuga de líquido na retina e, portanto, a hinchazón da mácula.
O edema macular diabético é uma hinchazón da retina que se produz em pacientes com diabetes devido à fuga de líquido dos vasos sanguíneos da mácula. A mácula é a parte da retina responsável pela visão fina. Quando a mácula se hincha de líquido, a visão central se torna borrosa.
Eylea demonstrou deter o crescimento dos novos vasos sanguíneos anômalos no olho que frequentemente sangram ou apresentam fugas de líquido. Eylea pode ajudar a estabilizar e, em muitos casos, a melhorar a perda de visão produzida pela DMAE exsudativa, OVCR, ORVR, EMD e NVC miópica.
2. O que necessita saber antes de que lhe administrem Eylea
Não lhe devem administrar Eylea
- se é alérgico a aflibercepte ou a algum dos outros componentes deste medicamento (incluídos na seção 6)
- se tem uma infecção ativa ou suspeita que possa ter uma infecção no olho ou ao seu redor (infecção ocular ou periocular)
- se padece uma inflamação grave do olho (indicada por dor ou enrubescimento).
Advertências e precauções
Consulte o seu médico antes de que lhe administrem Eylea:
- Se sofre glaucoma.
- Se tem antecedentes de visão de destelos de luz ou partículas flutuantes ou se de repente aumenta o tamanho e número de partículas flutuantes.
- Se lhe operaram ou tem programada uma cirurgia no olho nas quatro semanas anteriores ou nas quatro semanas seguintes.
- Se padece uma forma grave de OVCR ou bem ORVR (OVCR ou ORVR isquémicas), não está recomendado o tratamento com Eylea.
Além disso, é importante que saiba que:
- A segurança e eficácia de Eylea quando se administra em ambos os olhos ao mesmo tempo não se estudou e se se utiliza desta forma pode dar lugar a um maior risco de que se produzam efeitos adversos.
- As injeções de Eylea podem produzir um aumento da pressão dentro do olho (pressão intraocular) em alguns pacientes nos 60 minutos seguintes à injeção. O seu médico lhe realizará um seguimento após cada injeção.
- Se desenvolve uma infecção ou inflamação na parte interna do olho (endoftalmite) ou outras complicações, pode notar dor ou um aumento das molestias no olho, um agravamento do enrubescimento ocular, visão borrosa ou diminuída e aumento da sensibilidade à luz. É importante que todo sintoma que apareça se diagnostique e se trate o mais cedo possível.
- O seu médico comprovará se tem outros fatores de risco que possam aumentar a possibilidade de que se produza um desgarro ou um desprendimento das camadas posteriores do olho (desgarro ou desprendimento de retina, ou bem um desgarro ou desprendimento do epitélio pigmentário da retina) em cujo caso Eylea se lhe administrará com precaução.
- Eylea não se deve utilizar durante a gravidez, a menos que o benefício potencial supere o risco potencial para o feto.
- As mulheres em idade fértil devem utilizar métodos anticonceptivos eficazes durante o tratamento e durante pelo menos três meses mais após a última injeção de Eylea.
O uso sistémico de inibidores do VEGF, substâncias parecidas com as que contém Eylea, está potencialmente relacionado com o risco de bloqueio dos vasos sanguíneos por coágulos de sangue (acontecimentos tromboembólicos arteriais) que podem dar lugar a um infarto de miocárdio ou um acidente cerebrovascular. Após a injeção de Eylea no olho, existe um risco teórico de que se possam produzir estes acontecimentos. Os dados sobre a segurança do tratamento de pacientes com OVCR, ORVR, EMD e NVC miópica que sofreram um acidente cerebrovascular, um acidente cerebrovascular transitório (ataque isquémico transitório), ou bem um infarto de miocárdio nos últimos 6 meses são limitados. Se algum destes casos lhe aplica, se lhe administrará Eylea com precaução.
A experiência é só limitada no tratamento de:
- Pacientes com EMD devido à diabetes de tipo I.
- Pacientes diabéticos com valores médios de açúcar no sangue muito elevados (Hemoglobina glicosilada superior a 12%).
- Pacientes diabéticos com uma doença ocular provocada pela diabetes, conhecida como retinopatia diabética proliferativa.
Não existe experiência no tratamento de:
- Pacientes com infecções agudas.
- Pacientes com outras doenças oculares como desprendimento de retina ou buraco macular.
- Pacientes diabéticos com hipertensão não controlada.
- Pacientes não asiáticos com NVC miópica.
- Pacientes que foram tratados anteriormente por uma NVC miópica.
- Pacientes com danos fora da parte central da mácula (lesões extrafoveais) devido a uma NVC miópica.
Se algo do anterior lhe sucede, o seu médico terá em conta esta falta de informação no momento de o tratar com Eylea.
Crianças e adolescentes
Não se estudou o uso de Eylea em crianças e adolescentes menores de 18 anos para indicações distintas de retinopatia do prematuro (ROP).
Outros medicamentos e Eylea
Informa ao seu médico se está utilizando, utilizou recentemente ou pudesse ter que utilizar qualquer outro medicamento.
Gravidez e lactação
- As mulheres em idade fértil devem utilizar métodos anticonceptivos eficazes durante o tratamento e durante pelo menos três meses mais após a última injeção de Eylea.
- Não há experiência com o uso de Eylea em mulheres grávidas. Não se deve utilizar Eylea durante a gravidez a menos que o benefício potencial supere o risco potencial para o feto. Se está grávida ou tem intenção de ficar grávida, comente com o seu médico antes do tratamento com Eylea.
- Podem passar para o leite materno quantidades pequenas de Eylea. Desconhece-se os efeitos em recém-nascidos/bebês lactantes. Eylea não está recomendado durante a lactação. Se é uma mulher em período de lactação, comente com o seu médico antes do tratamento com Eylea.
Condução e uso de máquinas
Após a injeção de Eylea pode experimentar algumas alterações visuais transitórias. Não conduza nem use máquinas enquanto durarem estas alterações.
Eylea contém
- menos de 1 mmol de sódio (23 mg) por unidade de dose; isto é, essencialmente “exento de sódio”.
- 0,015 mg de polissorbato 20 em cada dose de 0,05 ml equivalente a 0,3 mg/ml. Os polissorbatos podem causar reações alérgicas. Informe ao seu médico se tem alguma alergia conhecida.
3. Como lhe será administrado Eylea
Eylea lhe será administrado por um médico com experiência na administração de injeções oculares, em condições assépticas (de limpeza e estéreis).
A dose recomendada é de 2 mg de aflibercepte (0,05 ml).
Eylea administra-se em forma de injeção no interior do olho (injeção intravítrea).
Antes da injeção, o seu médico utilizará um lavado ocular desinfetante para limpar cuidadosamente o seu olho para prevenir uma infecção. O seu médico também lhe administrará um anestésico local para reduzir ou prevenir qualquer dor que pudesse sentir com a injeção.
DMAE exsudativa
Os pacientes com DMAE exsudativa serão tratados com uma injeção mensal para as três primeiras doses, seguido de outra injeção após outros dois meses.
O seu médico decidirá então se o intervalo de tratamento entre as injeções pode manter-se cada dois meses ou estender-se gradualmente em intervalos de 2 ou 4 semanas se a sua doença se estabilizou. Se a sua doença piora, o intervalo entre as injeções pode acortar-se.
Não é necessário que o seu médico o visite entre injeções, a menos que o seu médico considere o contrário ou você experimente algum problema.
Edema macular secundário a OVR (de ramo ou central)
O seu médico determinará o programa de tratamento mais adequado para si. O seu tratamento será iniciado com uma série de injeções de Eylea administradas uma vez ao mês.
O intervalo entre duas injeções não deve ser inferior a um mês.
O seu médico poderá decidir interromper o tratamento com Eylea se não se beneficia do tratamento continuado.
O tratamento continuará com uma injeção uma vez ao mês até que a sua doença se estabilize. Pode necessitar de três ou mais injeções mensais.
O seu médico controlará a sua resposta ao tratamento e poderá continuar o tratamento, incrementando de forma gradual o intervalo entre as injeções para estabilizar a sua doença. Em caso de agravamento com um intervalo entre tratamentos mais longo, o seu médico reduzirá o intervalo entre injeções.
Em função da sua resposta ao tratamento, o seu médico decidirá o programa de seguimento e tratamento.
Edema macular diabético (EMD)
Os pacientes com EMD serão tratados com uma injeção mensal para as cinco primeiras doses consecutivas, e a continuación, uma injeção cada dois meses.
O intervalo entre tratamentos pode manter-se cada dois meses ou ajustar-se segundo a sua doença em função da exploração realizada pelo seu médico. O seu médico decidirá o programa de visitas de seguimento.
O seu médico poderá decidir a interrupção do tratamento com Eylea se comprovar que você não se beneficia do tratamento continuado.
Neovascularização coroide (NVC) miópica
Os pacientes com NVC miópica serão tratados com uma única injeção. Só receberá mais injeções se as explorações do seu médico revelam que a sua doença não melhorou.
O intervalo entre duas injeções não deve ser inferior a um mês.
Se a sua doença desaparece e depois regressa, o seu médico pode reiniciar o tratamento.
O seu médico decidirá sobre o programa de revisões de seguimento.
Apresentam-se instruções detalhadas para o uso ao final deste prospecto em “Como preparar e administrar Eylea a adultos”.
Se não lhe administrarem uma dose de Eylea
Pida uma nova consulta para que o examinem e lhe administrem a injeção.
Interrupção do tratamento com Eylea
Consulte o seu médico antes de interromper o tratamento.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Potencialmente poderiam produzir-se reações alérgicas(hipersensibilidade). Estas podem ser graves erequerem que se ponha em contacto com o seu médico imediatamente.
Com a administração de Eylea podem produzir-se alguns efeitos adversos que afetam os olhos que são devidos ao procedimento de injeção. Alguns podem ser graves, incluyendo cegueira, umainfecçãograve ou inflamação no interior do olho(endoftalmite), desprendimento, desgarro ou hemorragia da camada sensível à luz na parte posterior do olho(desprendimento ou desgarro da retina), enturbiação do cristalino(catarata), hemorragia no olho(hemorragia vítrea), desprendimento da substância semelhante a um gel que se encontra no interior do olho em contacto com a retina(desprendimento de vítreo) e aumento da pressão no interior do olho(ver seção 2). Estes efeitos adversos graves que afetam os olhos produziram-se em menos de 1 de 1.900 injeções durante os ensaios clínicos.
Se nota uma diminuição repentina da visão ou um aumento da dor e enrubescimento no olho após a injeção, consulte imediatamente o seu médico.
Lista dos efeitos adversos comunicados
A seguir inclui-se uma lista dos efeitos adversos comunicados como possivelmente relacionados com o procedimento de injeção ou com o medicamento. Não deve alarmar-se, porque pode que você não experimente nenhum deles. Consulte sempre com o seu médico acerca de qualquer suspeita de efeito adverso.
Efeitos adversos muito frequentes(podem afetar mais de 1 de cada 10 pessoas):
- degradação da visão
- sangramento na parte posterior do olho (hemorragia retiniana)
- sangue no olho devido ao sangramento de pequenos vasos sanguíneos nas camadas externas do olho
- dor ocular
Efeitos adversos frequentes(podem afetar até 1 de cada 10 pessoas):
- desprendimento ou desgarro de uma das camadas da parte posterior do olho que produzem destelos de luz com manchas flutuantes que por vezes progride para perda de visão (desgarro*/desprendimento do epitélio pigmentário da retina, desgarro/desprendimento da retina)
- *Efeitos adversos que se sabe estão associados à DMAE exsudativa; observados unicamente em pacientes com DMAE exsudativa.
- degeneração da retina (que causa alterações da visão)
- sangramento no olho (hemorragia vítrea)
- certas formas de enturbiação do cristalino (catarata)
- danos na camada superficial do globo ocular (a córnea)
- aumento da pressão no interior do olho
- manchas na visão (partículas flutuantes)
- desprendimento da substância semelhante a um gel que se encontra no interior do olho da retina (desprendimento vítreo, que resulta em destelos de luz com manchas flutuantes)
- sensação de ter algo dentro do olho
- aumento da produção de lágrimas
- inchação do párpado
- sangramento no local de injeção
- enrubescimento do olho
Efeitos adversos pouco frequentes(podem afetar até 1 de cada 100 pessoas):
- reações alérgicas (hipersensibilidade)**
- **Foram notificadas reações alérgicas como erupção, picazón (prurido), ronchas (urticária) e alguns casos de reações alérgicas (anafilácticas/anafilactoides) graves.
- inflamação ou infecção grave dentro do olho (endoftalmite)
- inflamação do íris ou de outras partes do olho (irite, uveíte, iridociclite, células flutuantes na câmara anterior)
- sensação anormal no olho
- irritação no párpado
- inchação da camada superficial do globo ocular (córnea)
Efeitos adversos raros(podem afetar até 1 de cada 1.000 pessoas)
- cegueira
- enturbiação do cristalino devido a lesão (catarata traumática)
- inflamação da substância semelhante a um gel que se encontra no interior do olho pus no olho
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis):
- inflamação da parte branca do olho associada com enrubescimento e dor (esclerite)
Nos ensaios clínicos observou-se um aumento da incidência de sangramento dos vasos sanguíneos pequenos nas camadas externas do olho (hemorragia conjuntival) em pacientes com DMAE exsudativa que recebiam tratamento com medicamentos anticoagulantes. Este aumento da incidência foi comparável nos pacientes tratados com ranibizumab e com Eylea.
O uso sistémico de inibidores do VEGF, substâncias semelhantes às contidas em Eylea, está potencialmente relacionado com o risco de formação de coágulos de sangue que bloqueiam os vasos sanguíneos (acontecimentos tromboembólicos arteriais) que podem dar lugar a um infarto de miocárdio ou uma embolia. Há um risco teórico de que possa produzir-se este tipo de acontecimentos após a injeção de Eylea no olho.
Como todas as proteínas terapêuticas, existe a possibilidade de uma reação imune (formação de anticorpos) com Eylea.
Comunicação de efeitos adversos
Se experimenta qualquer tipo de efeito adverso, consulte o seu médico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Eylea
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta após “CAD/EXP”. A data de validade é o último dia do mês que se indica.
- Conservar na geladeira (entre 2 ºC e 8 ºC). Não congelar.
- O blister sem abrir pode ser conservado fora da geladeira abaixo de 25 °C durante um máximo de 24 horas.
- Conservar no embalagem original para protegê-lo da luz.
- Os medicamentos não devem ser jogados pelos ralos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Eylea
- O princípio ativo é: aflibercepte. Uma seringa pré-carregada contém um volume extrair de pelo menos 0,09 ml, equivalente a pelo menos 3,6 mg de aflibercepte. Uma seringa pré-carregada fornece uma dose de 2 mg de aflibercepte em 0,05 ml.
- Os outros componentes são: polissorbato 20 (E 432), dihidrogenofosfato de sódio monohidratado (para o ajuste do pH), hidrogenofosfato de disódio heptahidratado (para o ajuste do pH), cloreto de sódio, sacarose, água para preparações injetáveis.
Ver “Eylea contém” na seção 2 para mais informações.
Aspecto do produto e conteúdo do envase
Eylea é uma solução injetável (injetável) em uma seringa pré-carregada. A solução é de incolora a amarelo pálido.
Envase com 1 seringa pré-carregada.
Título de autorização de comercialização
Bayer AG
51368 Leverkusen
Alemanha
Responsável pela fabricação
Bayer AG
Müllerstraße 178
13353 Berlim
Alemanha
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título de autorização de comercialização:
Bélgica / Bélgica / Bélgica Bayer SA-NV Tel: +32-(0)2-535 63 11 | Lituânia UAB Bayer Tel: +370-5-233 68 68 |
| Luxemburgo / Luxemburgo Bayer SA-NV Tel: +32-(0)2-535 63 11 |
República Checa Bayer s.r.o. Tel: +420-266 101 111 | Hungria Bayer Hungária KFT Tel: +36-1-487 4100 |
Dinamarca Bayer A/S Tlf: +45-45 235 000 | Malta Alfred Gera and Sons Ltd. Tel: +356-21 44 62 05 |
Alemanha Bayer Vital GmbH Tel: +49-(0)214-30 513 48 | Países Baixos Bayer B.V. Tel: +31–23-799 1000 |
Estônia Bayer OÜ Tel: +372-655 85 65 | Noruega Bayer AS Tlf: +47-23 13 05 00 |
Grécia Bayer Ελλάς ΑΒΕΕ Τηλ: +30-210-618 75 00 | Áustria Bayer Áustria Ges. m. b. H. Tel: +43-(0)1-711 460 |
Espanha Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polônia Bayer Sp. z o.o. Tel: +48-22-572 35 00 |
França Bayer HealthCare Tél (N° verde): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351-21-416 42 00 |
Croácia Bayer d.o.o. Tel: + 385-(0)1-6599 900 | Romênia SC Bayer SRL Tel: +40-(0)21-529 59 00 |
Irlanda Bayer Limited Tel: +353-(0)1-216 3300 | Eslovênia Bayer d. o. o. Tel: +386-(0)1-58 14 400 |
Islândia Icepharma hf. Sími: +354-540 80 00 | República Eslovaca Bayer, spol. s r.o. Tel: +421-(0)2-59 21 31 11 |
Itália Bayer S.p.A. Tel: +39-02-3978 1 | Finlândia Bayer Oy Puh/Tel: +358-(0)20-78521 |
Chipre NOVAGEM Limited Τηλ: +357-22-48 38 58 | Suécia Bayer AB Tel: +46-(0)8-580 223 00 |
Letônia SIA Bayer Tel: +371-67 84 55 63 |
Data da última revisão deste prospecto:
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu.
Se desejar informações locais, escaneie aqui para acessar o site https://www.pi.bayer.com/eylea1.
É incluído um código QR com o link para o prospecto.
<------------------------------------------------------------------------------------------------------------------
Esta informação é destinada apenas a profissionais do setor de saúde:
Como preparar e administrar Eylea a adultos
A seringa pré-carregada deve ser usada para o tratamento de um único olho.
Não abra o blister com a seringa pré-carregada estéril fora da sala limpa.
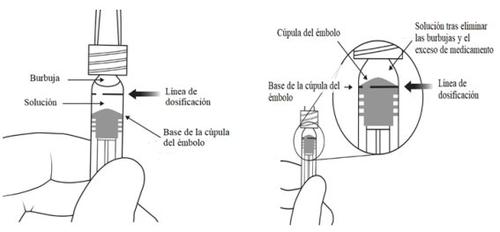
A seringa pré-carregada contém mais quantidade do que a dose recomendada de 2 mg de aflibercepte (equivalente a 0,05 ml). O excesso de volume deve ser eliminado antes da administração.
Antes da administração, a solução deve ser inspecionada visualmente para detectar a presença de partículas e/ou um cambio de cor ou qualquer mudança no aspecto físico. Se observar qualquer um deles, não use o medicamento.
O blister não aberto pode ser conservado fora da geladeira por baixo de 25 °C durante um máximo de 24 horas. Após a abertura do blister, proceda sob condições assépticas.
Para a injeção intravítrea, deve ser usada uma agulha de injeção de 30 G x ½ polegada (1,27 cm).
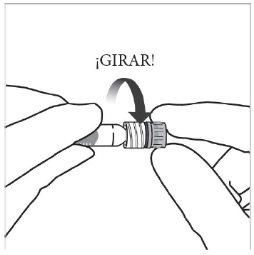
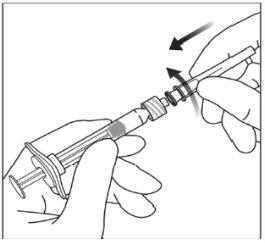
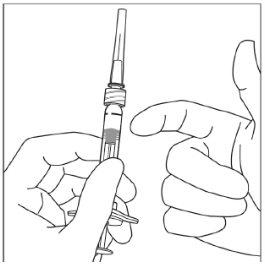
Instruções de uso da seringa pré-carregada:
| |
| |
|
|
| |
|
|
|
|
Nota:Esta posição exata do êmbolo é muito importante, porque uma posição incorreta do êmbolo pode fazer com que se administre mais ou menos da dose recomendada. | |
| |
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a regulamentação local. |
Prospecto: informação para os tutores de bebês nascidos prematuramente
Eylea 40 mg/ml solução injetável em seringa pré-carregada
aflibercepte
BEBÊS NASCIDOS PREMATURAMENTE
Leia todo o prospecto atentamente antes de que lhe administrem este medicamento ao bebê, porque contém informações importantes para você.
- Conserve este prospecto, pois pode ter que relê-lo.
- Se tiver alguma dúvida, consulte o médico do bebê.
- Se observar algum sintoma de efeitos adversos, consulte o médico do bebê, mesmo que se trate de sintomas e efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Eylea e para que é utilizado
- O que precisa saber antes de que lhe administrem Eylea ao bebê
- Como será administrado Eylea ao bebê
- Possíveis efeitos adversos
- Conservação de Eylea
- Conteúdo do envase e informação adicional
- O que é Eylea e para que é utilizado
Eylea é uma solução que é injetada no olho. Eylea pertence a um grupo de medicamentos denominados agentes antineovascularização. Contém o princípio ativo denominado aflibercepte.
Eylea é utilizado em bebês nascidos prematuramente para tratar um distúrbio ocular denominado retinopatia do prematuro (ROP). Os bebês com ROP têm um crescimento anômalo de novos vasos sanguíneos na parte posterior do olho (retina) induzido pelo fator de crescimento endotelial vascular (VEGF). Isso pode causar afetação da visão e, em casos graves, cegueira permanente.
Aflibercepte, o princípio ativo de Eylea, bloqueia a atividade de um grupo de fatores denominados fator de crescimento endotelial vascular A (VEGF-A) e fator de crescimento placentário (PlGF).
Eylea demonstrou parar o crescimento dos novos vasos sanguíneos anômalos no olho que frequentemente apresentam fugas de líquido ou sangram. Eylea pode ajudar a estabilizar e, em muitos casos, a melhorar a perda de visão produzida pela ROP.
- O que precisa saber antes de que lhe administrem Eylea ao bebê
Não lhe devem administrar Eylea ao bebê
- se é alérgicoa aflibercepte ou a algum dos outros componentes deste medicamento (incluídos na seção 6)
- se tem uma infecção ativa ou suspeita que possa ter uma infecção no olho ou ao seu redor (infecção ocular ou periocular)
- se padece uma inflamação grave do olho (indicada por dor ou vermelhidão).
Advertências e precauções
Consulte o médico do bebê antes de que lhe administrem Eylea
- Se operaram o bebê ou tem programada uma cirurgia no olho nas quatro semanas anteriores ou nas quatro semanas seguintes.
Além disso, é importante que saiba que
- As injeções de Eylea podem produzir um aumento da pressão dentro do olho (pressão intraocular) em alguns pacientes nos 60 minutos seguintes à injeção. O médico do bebê fará um acompanhamento após cada injeção.
- Se o bebê desenvolver uma infecção ou inflamação na parte interna do olho (endoftalmitis) ou outras complicações, o bebê pode apresentar vermelhidão/irritação do olho, secreção ocular, inchaço do párpadoe aumento da sensibilidade à luz. É importante que todo sintoma que apareça seja diagnosticado e tratado o mais rápido possível.
Informe imediatamente ao médico do bebê se apresentar os sinais ou sintomas descritos.
- O médico do bebê verificará se tem outros fatores de risco que possam aumentar a possibilidade de que se produza um desgarro ou um desprendimento de uma das camadas posteriores do olho (desgarro ou desprendimento de retina) no qual caso Eylea será administrado com precaução.
O uso sistêmico de inibidores do VEGF, substâncias semelhantes às que contém Eylea, está potencialmente relacionado com o risco de bloqueio dos vasos sanguíneos por coágulos de sangue (acontecimentos tromboembólicos arteriais) que podem dar lugar a um infarto de miocárdio ou um acidente cerebrovascular. Após a injeção de Eylea no olho, existe um risco teórico de que se possam produzir esses acontecimentos.
Não existe experiência no tratamento de:
- Pacientes com infecções agudas.
- Pacientes com outras doenças oculares como desprendimento de retina ou buraco macular.
Se algo do anterior acontecer ao bebê, o médico do bebê terá em conta essa falta de informação no momento de tratar o bebê com Eylea.
Outros medicamentos e Eylea
Informe o médico do bebê se este está recebendo, recebeu recentemente ou pudesse ter que receber qualquer outro medicamento.
Eylea contém
- menos de 1 mmol de sódio (23 mg) por unidade de dose; isto é, essencialmente “exento de sódio”.
- 0,003 mg de polissorbato 20 em cada dose de 0,01 ml equivalente a 0,3 mg/ml. Os polissorbatos podem causar reações alérgicas. Informe ao seu médico se tem alguma alergia conhecida.
- Como será administrado Eylea ao bebê
Eylea será administrado ao bebê nos olhos por um médico com experiência na administração de injeções oculares, em condições assépticas (de limpeza e estéreis).
A dose recomendada é de 0,4 mg de aflibercepte (0,01 ml).
Eylea é administrado em forma de injeção no interior do olho do bebê (injeção intravítrea).
Antes da injeção, o médico do bebê utilizará um lavado ocular desinfetante para limpar cuidadosamente o olho do bebê para prevenir uma infecção. O médico do bebê também administrará um anestésico local para reduzir ou prevenir qualquer dor que o bebê possa sentir com a injeção.
O tratamento é iniciado com uma injeção única por olho e pode ser administrado no segundo olho no mesmo dia. O médico do bebê controlará o estado do (dos) olho(s) do bebê. Dependendo de como o bebê responde ao tratamento, o médico do bebê decidirá se é necessário um tratamento adicional e quando deve ser administrado. O intervalo de tratamento entre as duas doses injetadas no mesmo olho deve ser de pelo menos 4 semanas.
São apresentadas instruções detalhadas para o uso no final deste prospecto em “Como preparar e administrar Eylea a bebês prematuros”.
Interrupção do tratamento com Eylea
Se está considerando interromper o tratamento com Eylea, fale com o médico do bebê na sua próxima consulta. O médico do bebê aconselhará e decidirá durante quanto tempo se deve tratar o bebê com Eylea.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao médico do bebê.
- Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Os efeitos adversos comunicados em mais de um bebê nascido prematuramente foram
- desprendimento da camada situada na parte posterior do olho(desprendimento de retina)
- sangramento na parte posterior do olho(hemorragia retiniana)
- olho injetado em sanguedevido a hemorragia procedente de pequenos vasos sanguíneos nas camadas externas do olho (hemorragia conjuntival)
- sangramento no local de injeção(hemorragia no local de injeção)
- aumento da pressão ocular
- inchaço do párpado(edema palpebral)
A seguir são indicados outros efeitos adversosque foram observados com Eylea em adultos. Esses efeitos adversos também podem ocorrer em bebês nascidos prematuramente:
- reações alérgicas(hipersensibilidade).
Estas podem ser graves e requerer que se ponha em contacto com o médico do bebê imediatamente.
Os efeitos adversos que afetam os olhos devido ao procedimento de injeção podem ser graves, incluindo
- cegueira
- infecçãograve ou inflamaçãono interior do olho (endoftalmitis)
- desprendimento, desgarro ou hemorragiada camada sensível à luz na parte posterior do olho (desprendimento ou desgarro da retina)
- enturbiação do cristalino(catarata)
- hemorragia no olho(hemorragia vítrea)
- desprendimentoda substância semelhante a um gel que se encontra no interior do olho em contato com a retina (desprendimento de vítreo)
- aumento da pressãono interior do olho (aumento da pressão intraocular), ver seção 2.
Esses efeitos adversos graves que afetam os olhos ocorreram em menos de 1 de 1.900 injeções durante os ensaios clínicos em adultos.
É importante identificar e tratar o mais rápido possível os efeitos adversos graves, como uma infecção no interior do olho ou um desprendimento de retina.
Informe imediatamente ao médico do bebê seobservar sintomas no olho do bebê após a injeção, por exemplo
- vermelhidão/irritação
- secreção ocular
- inchaço do párpado
- aumento da sensibilidade à luz
A seguir são descritos outros efeitos adversos observados em adultos.
Lista dos efeitos adversos comunicados
A seguir é incluída uma lista dos efeitos adversos comunicados como possivelmente relacionados com o procedimento de injeção ou com o medicamento. Não deve alarmar-se, pois pode que o bebê não experimente nenhum deles. Consulte sempre com o médico do bebê sobre qualquer suspeita de efeito adverso.
Efeitos adversos muito frequentes(podem afetar mais de 1 de cada 10 pessoas):
- degradação da visão
- sangramento na parte posterior do olho (hemorragia retiniana)
- sangue no olho devido ao sangramento de pequenos vasos sanguíneos nas camadas externas do olho
- dor ocular
Efeitos adversos frequentes(podem afetar até 1 de cada 10 pessoas):
- desprendimento ou desgarro de uma das camadas da parte posterior do olho que produzem destellos de luz commanchas flutuantes que por vezes progridem para perda de visão (desgarro/desprendimento do epitélio pigmentar da retina, desgarro/desprendimento da retina)
- *Efeitos adversos que se sabe estão associados à DMAE exsudativa; observados unicamente em pacientes com DMAE exsudativa.
- degeneração da retina que causa alterações da visão
- sangramento no olho (hemorragia vítrea)
- certas formas de turvação do cristalino (catarata)
- danos na camada superficial do globo ocular (a córnea)
- aumento da pressão no interior do olho
- manchas na visão (partículas flutuantes)
- desprendimento da substância semelhante a um gel que se encontra no interior do olho da retina (desprendimento vítreo, que resulta em destelos de luz com manchas flutuantes)
- sensação de ter algo dentro do olho
- aumento da produção de lágrimas
- inchação da pálpebra
- sangramento no local de injeção
- vermelhidão do olho
Efeitos adversos pouco frequentes(podem afetar até 1 de cada 100 pessoas):
- reações alérgicas (hipersensibilidade)**
- ** Foram notificadas reações alérgicas como erupção, prurido (coceira), urticária e alguns casos de reações alérgicas (anafiláticas/anafilactoides) graves.
- inflamação ou infecção grave dentro do olho (endoftalmite)
- inflamação do íris ou de outras partes do olho (irite, uveíte, iridociclite, células flutuantes na câmara anterior)
- sensação anormal no olho
- irritação na pálpebra
- inchação da camada superficial do globo ocular (córnea)
Efeitos adversos raros(podem afetar até 1 de cada 1.000 pessoas):
- cegueira
- turvação do cristalino devido a lesão (catarata traumática)
- inflamação da substância semelhante a um gel que se encontra no interior do olho
- pus no olho
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis):
- inflamação da parte branca do olho associada com vermelhidão e dor (esclerite)
O uso de inibidores do VEGF por via sistémica, substâncias semelhantes às contidas em Eylea, está potencialmente relacionado com o risco de formação de coágulos de sangue que bloqueiam os vasos sanguíneos (eventos tromboembólicos arteriais) que podem produzir um ataque ao coração ou uma embolia. Há um risco teórico de que possa ocorrer este tipo de eventos após a injeção de Eylea no olho.
Assim como com todas as proteínas terapêuticas, existe a possibilidade de uma reação imune (formação de anticorpos) com Eylea.
Se tiver alguma dúvida sobre algum efeito adverso, pergunte ao médico do bebê.
Comunicação de efeitos adversos
Se observar qualquer tipo de efeito adverso no bebê, consulte o médico do bebê, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
- Conservação de Eylea
- Manter este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta após “CAD/EXP”. A data de validade é o último dia do mês que se indica.
- Conservar em geladeira (entre 2 °C e 8 °C). Não congelar.
- O blíster não aberto pode ser conservado fora da geladeira por debaixo de 25 °C durante um máximo de 24 horas.
- Conservar no embalagem original para protegê-lo da luz.
- Os medicamentos não devem ser jogados pelos deságues nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
- Conteúdo do envase e informações adicionais
Composição de Eylea
- O princípio ativo é: aflibercepte. Uma seringa pré-carregada contém um volume extráível de pelo menos 0,09 ml, equivalente a pelo menos 3,6 mg de aflibercepte. Uma seringa pré-carregada fornece uma dose única de 0,4 mg de aflibercepte em 0,01 ml.
- Os demais componentes são: polissorbato 20 (E 432), dihidrogenofosfato de sódio monohidratado (para o ajuste do pH), hidrogenofosfato de disódio heptahidratado (para o ajuste do pH), cloreto de sódio, sacarose, água para preparações injetáveis.
Ver “Eylea contém” na seção 2 para mais informações.
Aspecto do produto e conteúdo do envase
Eylea é uma solução injetável (injetável) em uma seringa pré-carregada. A solução é de incolora a amarelo pálido.
Envase com 1 seringa pré-carregada.
Titular da autorização de comercialização
Bayer AG
51368 Leverkusen
Alemanha
Responsável pela fabricação
Bayer AG
Müllerstraße 178
13353 Berlim
Alemanha
Podem solicitar mais informações respeito a este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica / Bélgica / Bélgica Bayer SA-NV Tel: +32-(0)2-535 63 11 | Lituânia UAB Bayer Tel: +370-5-233 68 68 |
| Luxemburgo / Luxemburgo Bayer SA-NV Tel: +32-(0)2-535 63 11 |
República Tcheca Bayer s.r.o. Tel: +420-266 101 111 | Hungria Bayer Hungária KFT Tel: +36-1-487 4100 |
Dinamarca Bayer A/S Tlf: +45-45 235 000 | Malta Alfred Gera and Sons Ltd. Tel: +356-21 44 62 05 |
Alemanha Bayer Vital GmbH Tel: +49-(0)214-30 513 48 | Países Baixos Bayer B.V. Tel: +31–23-799 1000 |
Estônia Bayer OÜ Tel: +372-655 85 65 | Noruega Bayer AS Tlf: +47-23 13 05 00 |
Grécia Bayer Ελλάς ΑΒΕΕ Τηλ: +30-210-618 75 00 | Áustria Bayer Áustria Ges. m. b. H. Tel: +43-(0)1-711 460 |
Espanha Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polônia Bayer Sp. z o.o. Tel: +48-22-572 35 00 |
França Bayer HealthCare Tél (N° verde): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351-21-416 42 00 |
Croácia Bayer d.o.o. Tel: + 385-(0)1-6599 900 | Romênia SC Bayer SRL Tel: +40-(0)21-529 59 00 |
Irlanda Bayer Limited Tel: +353-(0)1-216 3300 | Eslovênia Bayer d. o. o. Tel: +386-(0)1-58 14 400 |
Islândia Icepharma hf. Sími: +354-540 80 00 | República Eslovaca Bayer, spol. s r.o. Tel: +421-(0)2-59 21 31 11 |
Itália Bayer S.p.A. Tel: +39-02-3978 1 | Finlândia Bayer Oy Puh/Tel: +358-(0)20-78521 |
Chipre NOVAGEM Limited Τηλ: +357-22-48 38 58 | Suécia Bayer AB Tel: +46-(0)8-580 223 00 |
Letônia SIA Bayer Tel: +371-67 84 55 63 |
Data da última revisão desteprospecto:
Outras fontes de informação
A informação detalhada deste medicamento está disponível na página web da Agência Europeia de Medicamentos: https://www.ema.europa.eu.
Se desejar informação local, escaneie aqui para acessar o site https://www.pi.bayer.com/eylea1.
É incluído um código QR com o link para o prospecto.
<------------------------------------------------------------------------------------------------------------------
Estainformação está destinada unicamente a profissionais de saúde:
Como preparar e administrar Eylea a recém-nascidos pré-termo
A seringa pré-carregada deve ser utilizada para o tratamento de um único olho. A extração de múltiplas doses de uma seringa pré-carregada pode aumentar o risco de contaminação e de posterior infecção.
Não abra o blíster pré-carregado estéril fora da sala limpa. A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a normativa local.
A seringa pré-carregada contém mais quantidade do que a dose recomendada de 0,4 mg de aflibercepte (equivalente a 0,01 ml). Para o tratamento de recém-nascidos pré-termo, deve-se usar o dispositivo dosificador pediátrico PICLEO em combinação com a seringa pré-carregada para administrar uma dose única de 0,4 mg de aflibercepte (equivalente a 0,01 ml). Ver a seguinte seção “Instruções de uso da seringa pré-carregada”.
Antes da administração, a solução deve ser inspecionada visualmente para detectar a presença de partículas e/ou um cambio de cor ou qualquer cambio no aspecto físico. Se observar qualquer um deles, não utilize o medicamento.
O blíster não aberto pode ser conservado fora da geladeira por debaixo de 25 °C durante um máximo de 24 horas. Após a abertura do blíster, proceda sob condições assépticas.
Para a injeção intravítrea deve-se usar uma agulha de injeção de 30 G x ½ polegada (1,27 cm).
Instruções de uso da seringa pré-carregada:
Para preparar a seringa pré-carregada para a administração a recém-nascidos pré-termo, siga os passos 1 e 2 descritos mais adiante e, a seguir, siga as instruções de uso incluídas no envase do dispositivo dosificador pediátrico PICLEO.
- Quando estiver preparado para administrar Eylea, abra a caixa e extraia o blíster esterilizado. Despegue cuidadosamente a lâmina do blíster, assegurando a esterilidade de seu conteúdo. Mantenha a seringa na bandeja estéril até que esteja listo para o ensamblagem.
- Utilizando uma técnica asséptica, extraia a seringa do envase do blíster esterilizado.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a EYLEA 40 mg/ml Solução injetável em seringa pré-carregadaForma farmacêutica: INJETÁVEL, 40 mg/mlSubstância ativa: afliberceptFabricante: Sandoz GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 40 mg/mlSubstância ativa: afliberceptFabricante: Sandoz GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 40 mg/mlSubstância ativa: afliberceptFabricante: Celltrion Healthcare Hungary Kft.Requer receita médica
Alternativas a EYLEA 40 mg/ml Solução injetável em seringa pré-carregada noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a EYLEA 40 mg/ml Solução injetável em seringa pré-carregada em Ukraine
Médicos online para EYLEA 40 mg/ml Solução injetável em seringa pré-carregada
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de EYLEA 40 mg/ml Solução injetável em seringa pré-carregada – sujeita a avaliação médica e regras locais.