Elocta 1000 UI pó e solvente para solução injetável

Pergunte a um médico sobre a prescrição de Elocta 1000 UI pó e solvente para solução injetável

Como usar Elocta 1000 UI pó e solvente para solução injetável
Introdução
Prospecto: informação para o utilizador
ELOCTA 250 UI pó e dissolvente para solução injectável
ELOCTA 500 UI pó e dissolvente para solução injectável
ELOCTA 750 UI pó e dissolvente para solução injectável
ELOCTA 1000 UI pó e dissolvente para solução injectável
ELOCTA 1500 UI pó e dissolvente para solução injectável
ELOCTA 2000 UI pó e dissolvente para solução injectável
ELOCTA 3000 UI pó e dissolvente para solução injectável
ELOCTA 4000 UI pó e dissolvente para solução injectável
efmoroctocog alfa (factor VIII de coagulação recombinante)
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi-lhe prescrito apenas a si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é ELOCTA e para que é utilizado
- O que precisa saber antes de começar a usar ELOCTA
- Como usar ELOCTA
- Efeitos adversos possíveis
- Conservação de ELOCTA
- Conteúdo do envase e informações adicionais
1. O que é ELOCTA e para que é utilizado
ELOCTA contém o princípio ativo efmoroctocog alfa, um factor VIII de coagulação recombinante, proteína de fusão Fc. O factor VIII é uma proteína produzida de forma natural pelo corpo e é necessária para que o sangue forme coágulos e detenha as hemorragias. ELOCTA é um medicamento utilizado para o tratamento e a prevenção das hemorragias nos pacientes de todas as idades com hemofilia A (um distúrbio hemorrágico hereditário causado por uma deficiência do factor VIII).
ELOCTA é preparado mediante tecnologia recombinante sem a adição de qualquer componente de origem humana ou animal no processo de fabricação.
Como actua ELOCTA
Nos pacientes com hemofilia A, o factor VIII está ausente ou não funciona adequadamente. ELOCTA é utilizado para substituir o factor VIII ausente ou deficiente. ELOCTA aumenta as concentrações de factor VIII no sangue e corrige temporariamente a tendência a sofrer hemorragias.
2. O que precisa saber antes de começar a usar ELOCTA
Não use ELOCTA:
- se é alérgico ao efmoroctocog alfa ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar ELOCTA.
- Existe uma pequena possibilidade de que sofra uma reacção anafiláctica (uma reacção alérgica grave e repentina) a ELOCTA. Entre os sinais das reacções alérgicas incluem-se picazón generalizado, erupções, sensação de opressão no peito, dificuldade para respirar e pressão arterial baixa. Se aparecer algum destes sintomas, interrompa imediatamente a injeção e entre em contacto com o seu médico.
- A formação de inibidores (anticorpos) é uma complicação conhecida que pode ocorrer durante o tratamento com todos os medicamentos compostos por factor VIII. Estes inibidores, especialmente em grandes quantidades, impedem que o tratamento funcione corretamente, por isso serão supervisionados cuidadosamente si e o seu filho por si desenvolverem ditos inibidores. Se a hemorragia ou a do seu filho não estiver a ser controlada com ELOCTA, consulte o seu médico imediatamente.
Eventos cardiovasculares
Se tem uma doença do coração ou se encontra em risco de sofrê-la, tenha especial cuidado ao utilizar medicamentos com factor VIII e consulte o seu médico.
Complicações relacionadas com o catéter
Se necessita de um dispositivo de acesso venoso central (DAVC), deve ter-se em conta o risco de complicações relacionadas com o DAVC, incluindo as infecções locais, a presença de bactérias no sangue e a trombose no local de inserção do catéter.
Documentação
Recomendamos-lhe encarecidamente que cada vez que se administre ELOCTA, se anotem o nome e o número de lote do produto.
Outros medicamentos e ELOCTA
Informa o seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento.
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Condução e uso de máquinas
Não se observaram efeitos sobre a capacidade para conduzir ou utilizar máquinas.
ELOCTA contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por frasco; isto é, é essencialmente “isento de sódio”.
No entanto, dependendo do seu peso corporal e da dose, pode receber mais de um frasco, o que deve ser tido em conta se segue uma dieta pobre em sódio.
3. Como usar ELOCTA
O tratamento com ELOCTA será iniciado por um médico com experiência no cuidado de pacientes com hemofilia. Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico (ver secção Instruções de preparação e administração). Em caso de dúvida, consulte de novo o seu médico, farmacêutico ou enfermeiro.
ELOCTA é administrado mediante injeção numa veia. O seu médico calculará a sua dose de ELOCTA (em Unidades Internacionais ou “UI”), dependendo das suas necessidades individuais de tratamento de substituição do factor VIII e de se é utilizado para a prevenção ou o tratamento das hemorragias. Consulte o seu médico se acredita que não está a controlar as hemorragias com a dose que recebe.
Com que frequência necessitará de uma injeção, dependerá do grau de eficácia que ELOCTA estiver a mostrar consigo. O seu médico realizará os testes de laboratório pertinentes para se certificar de que tem concentrações adequadas de factor VIII no sangue.
Tratamento das hemorragias
A dose de ELOCTA é calculada em função do seu peso corporal e das concentrações de factor VIII que se desejam alcançar. As concentrações objectivo de factor VIII dependem da gravidade e da localização da hemorragia.
Prevenção das hemorragias
A dose habitual de ELOCTA é de 50 UI por kg de peso corporal, administradas cada 3 a 5 dias. O seu médico pode ajustar a dose num intervalo compreendido entre 25 e 65 UI por kg de peso corporal. Em alguns casos, especialmente nos pacientes mais jovens, pode ser necessário usar intervalos de dosagem mais curtos ou doses maiores.
Uso em crianças e adolescentes
ELOCTA pode ser utilizado em crianças e adolescentes de todas as idades. Nas crianças menores de 12 anos, podem ser necessárias doses mais altas ou injeções mais frequentes.
Se usa mais ELOCTA do que deve
Informa o seu médico o mais breve possível. Siga exactamente as instruções de administração de ELOCTA indicadas pelo seu médico. Em caso de dúvida, consulte de novo o seu médico, farmacêutico ou enfermeiro.
Se esqueceu de usar ELOCTA
Não tome uma dose dupla para compensar as doses esquecidas. Tome a sua dose assim que se lembrar e depois retome a sua pauta normal de dosagem. Se não tem a certeza do que deve fazer, consulte o seu médico ou farmacêutico.
Se interromper o tratamento com ELOCTA
Não interrompa o tratamento com ELOCTA sem consultar o seu médico. Se interromper o tratamento com ELOCTA, é possível que já não esteja protegido contra as hemorragias ou que uma hemorragia já existente não se detenha.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Se se produzirem reacções alérgicas graves e repentinas (reacção anafiláctica), a injeção deve ser interrompida imediatamente. Deve entrar em contacto com o seu médico imediatamente se apresentar algum dos seguintes sintomas de reacções alérgicas: inchação da face, erupções, picazón generalizado, erupções, sensação de opressão no peito, dificuldade para respirar, escozor e picadas no local da injeção, arrepios, sofocos, dor de cabeça, pressão arterial baixa, sensação geral de malestar, náuseas, agitação e batimento cardíaco rápido, sensação de tontura ou perda de consciência.
Nas crianças sem tratamento prévio com medicamentos compostos por factor VIII podem produzir-se anticorpos inibidores (ver secção 2) muito frequentemente (mais de 1 de cada 10 pacientes); no entanto, nos pacientes que receberam tratamento prévio com factor VIII (mais de 150 dias de tratamento), o risco é pouco frequente (menos de 1 de cada 100 pacientes). Se isto acontecer, os medicamentos podem deixar de funcionar corretamente e pode sofrer uma hemorragia persistente. Nesse caso, contacte o seu médico imediatamente.
Com este medicamento podem aparecer os seguintes efeitos adversos.
Efeitos adversos pouco frequentes (podem afectar até 1 de cada 100 pessoas)
Dor de cabeça, tontura, alterações do gosto, batimento cardíaco lento, pressão arterial alta, sofocos, dor vascular após a injeção, tosse, dor abdominal baixa, erupções cutâneas, erupções papulares, trombose relacionada com o dispositivo, inchação articular, dor muscular, dor de costas, dor articular, malestar geral, dor no peito, sensação de frio, sensação de calor e pressão arterial baixa.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de ELOCTA
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta do frasco após “CAD/EXP”. A data de validade é o último dia do mês que se indica. Não utilize este medicamento se foi conservado a temperatura ambiente durante mais de 6 meses.
Conservar em frigorífico (entre 2 °C - 8 °C).
Não congelar.
Conservar no embalagem original para protegê-lo da luz.
Alternativamente, ELOCTA pode ser conservado a temperatura ambiente (até 30 °C) durante um período único que não ultrapasse os 6 meses. Anote na caixa a data em que ELOCTA foi retirado do frigorífico e deixado a temperatura ambiente. Após a conservação a temperatura ambiente, o medicamento não deve ser reintroduzido no frigorífico.
Uma vez preparado ELOCTA, deve utilizá-lo imediatamente. Se não puder usar a solução preparada de ELOCTA de imediato, deve utilizá-la num prazo máximo de 6 horas. Não refrigere a solução preparada. Proteja a solução preparada da luz solar directa.
A solução preparada deve ser transparente a ligeiramente opalescente e incolor. Não utilize este medicamento se observar que está turvo ou contém partículas visíveis.
Elimine adequadamente qualquer resto de solução não utilizada. Os medicamentos não devem ser deitados fora por os esgotos nem para o lixo. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informações adicionais
Composição de ELOCTA
- O princípio ativo é efmoroctocog alfa (fator VIII de coagulação recombinante, proteína de fusão Fc). Cada frasco de ELOCTA contém nominalmente 250, 500, 750, 1.000, 1.500, 2.000, 3.000 ou 4.000 UI de efmoroctocog alfa.
- Os demais componentes são sacarose, cloreto de sódio, L-histidina, cloreto de cálcio dihidratado, polissorbato 20, hidróxido de sódio, ácido clorídrico e água para preparações injetáveis. Ver seção 2 se segue uma dieta pobre em sódio.
Aspecto do produto e conteúdo do frasco
ELOCTA é apresentado sob a forma de pó e diluente para solução injetável. O pó é um pó ou torta de cor branca a esbranquiçada. O diluente fornecido para a preparação da solução injetável é uma solução transparente e incolor. Após a preparação, a solução para injetar é transparente a ligeiramente opalescente e incolor.
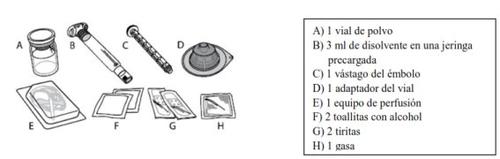
Cada frasco de ELOCTA contém 1 frasco de pó, 3 ml de diluente em uma seringa pré-carregada, 1 haste do êmbolo, 1 adaptador do frasco, 1 equipamento de perfusão, 2 compressas com álcool, 2 tiras e 1 gaze.
Título da autorização de comercialização e responsável pela fabricação
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Estocolmo,
Suécia
Data da última revisão deste prospecto: 01/2021
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
Vire o prospecto para consultar as instruções de preparação e administração
Instruções de preparação e administração
ELOCTA é administrado por injeção intravenosa (IV) após dissolver o pó injetável com o diluente fornecido na seringa pré-carregada. O frasco de ELOCTA contém:
ELOCTA não deve ser misturado com outras soluções injetáveis ou para perfusão.
Lave as mãos antes de abrir o frasco.
Preparação:
|
|
|
|
|
|
|
|
|
|
|
Não o agite. |
|
|
|
Nota: se usar mais de um frasco de ELOCTA por injeção, cada frasco deve ser preparado separadamente de acordo com as instruções anteriores (passos 1 a 13) e a seringa de diluente deve ser removida, deixando o adaptador do frasco colocado em sua posição. Pode-se usar uma única seringa luer lock maior para extrair o conteúdo preparado de cada um dos frascos. |
Nota: se a solução não for usada imediatamente, a cápsula de fechamento da seringa deve ser recolocada cuidadosamente sobre a ponta da seringa. Não toque a ponta da seringa nem o interior da cápsula de fechamento. Após a preparação, ELOCTA pode ser armazenado a temperatura ambiente durante um máximo de 6 horas antes da administração. Uma vez transcorrido este tempo, a solução preparada de ELOCTA deve ser descartada. Proteja-a da luz solar direta. |
Administração (injeção intravenosa):
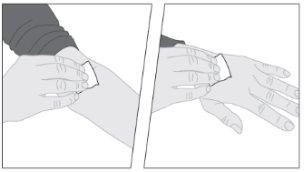
ELOCTA deve ser administrado usando o equipamento de perfusão (E) fornecido no frasco.
|
|
|
|
|
|
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a Elocta 1000 UI pó e solvente para solução injetávelForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1500 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1000 UI - após reconstituição em 2 ml de água para injetáveis, a dose é de 500 UI/mlSubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médica
Alternativas a Elocta 1000 UI pó e solvente para solução injetável noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a Elocta 1000 UI pó e solvente para solução injetável em Polónia
Alternativa a Elocta 1000 UI pó e solvente para solução injetável em Ukraine
Médicos online para Elocta 1000 UI pó e solvente para solução injetável
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de Elocta 1000 UI pó e solvente para solução injetável – sujeita a avaliação médica e regras locais.




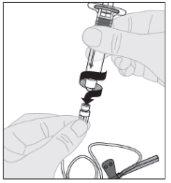
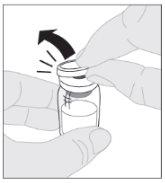 Coloque o frasco sobre uma superfície limpa e plana. Retire a cápsula de fechamento de plástico do frasco de ELOCTA.
Coloque o frasco sobre uma superfície limpa e plana. Retire a cápsula de fechamento de plástico do frasco de ELOCTA.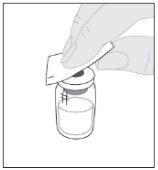 Limpe a parte superior do frasco com uma das compressas com álcool (F) fornecidas no frasco e deixe que seque ao ar. Não toque a parte superior do frasco nem permita que entre em contato com nada uma vez que o tenha limpo.
Limpe a parte superior do frasco com uma das compressas com álcool (F) fornecidas no frasco e deixe que seque ao ar. Não toque a parte superior do frasco nem permita que entre em contato com nada uma vez que o tenha limpo.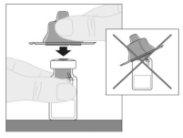
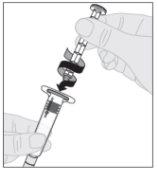 Acople a haste do êmbolo (C) à seringa de diluente inserindo a ponta da haste no orifício do êmbolo da seringa. Gire a haste do êmbolo firmemente no sentido horário até que fique bem assentada no êmbolo da seringa.
Acople a haste do êmbolo (C) à seringa de diluente inserindo a ponta da haste no orifício do êmbolo da seringa. Gire a haste do êmbolo firmemente no sentido horário até que fique bem assentada no êmbolo da seringa.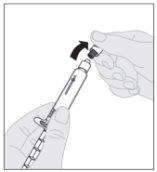 Desprenda a cápsula de fechamento de segurança inviolável de plástico branco da seringa de diluente dobrando-a pela cápsula de fechamento de perfuração até que se rompa. Deixe a cápsula de fechamento à parte colocando-a com a parte de cima mirando para baixo sobre uma superfície plana. Não toque o interior da cápsula de fechamento nem a ponta da seringa.
Desprenda a cápsula de fechamento de segurança inviolável de plástico branco da seringa de diluente dobrando-a pela cápsula de fechamento de perfuração até que se rompa. Deixe a cápsula de fechamento à parte colocando-a com a parte de cima mirando para baixo sobre uma superfície plana. Não toque o interior da cápsula de fechamento nem a ponta da seringa.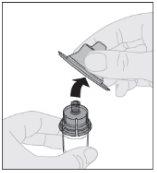
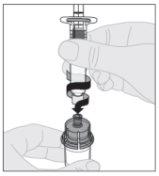 Conecte a seringa de diluente ao adaptador do frasco inserindo a ponta da seringa no orifício do adaptador. Empurre firmemente e gire a seringa no sentido horário até que fique bem conectada.
Conecte a seringa de diluente ao adaptador do frasco inserindo a ponta da seringa no orifício do adaptador. Empurre firmemente e gire a seringa no sentido horário até que fique bem conectada.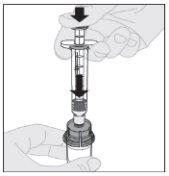 Pressione lentamente para baixo a haste do êmbolo para injetar todo o diluente no frasco de ELOCTA.
Pressione lentamente para baixo a haste do êmbolo para injetar todo o diluente no frasco de ELOCTA.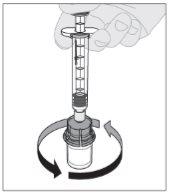
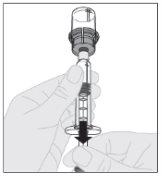 Certificando-se de que a haste do êmbolo da seringa ainda esteja completamente pressionada para baixo, vire o frasco. Puxe lentamente a haste do êmbolo para transferir toda a solução para o interior da seringa através do adaptador do frasco.
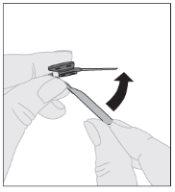
Certificando-se de que a haste do êmbolo da seringa ainda esteja completamente pressionada para baixo, vire o frasco. Puxe lentamente a haste do êmbolo para transferir toda a solução para o interior da seringa através do adaptador do frasco.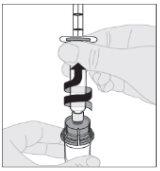 Desacople a seringa do adaptador do frasco puxando suavemente o frasco enquanto o gira no sentido anti-horário.
Desacople a seringa do adaptador do frasco puxando suavemente o frasco enquanto o gira no sentido anti-horário.