CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO


Como usar CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o utilizador
Cabazitaxel Dr. Reddys 60 mg concentrado e dissolvente para solução para perfusão EFG
cabazitaxel
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeira.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeira, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Cabazitaxel Dr. Reddys e para que é utilizado
- O que precisa saber antes de que lhe administrem Cabazitaxel Dr. Reddys
- Como usar Cabazitaxel Dr. Reddys
- Possíveis efeitos adversos
- Conservação de Cabazitaxel Dr. Reddys
- Conteúdo do envase e informação adicional
1. O que é Cabazitaxel Dr. Reddys e para que é utilizado
O nome do seu medicamento é Cabazitaxel Dr. Reddys. A sua denominação comum é cabazitaxel. Pertence a um grupo de medicamentos denominado “taxanos”, utilizados para tratar cancros.
Este medicamento é utilizado para o tratamento do cancro da próstata que progrediu após ter recebido outra quimioterapia. Actua detendo o crescimento das células e a sua multiplicação.
Como parte do seu tratamento, tomará também todos os dias um corticosteroide (prednisona ou prednisolona), por via oral. Peça informações ao seu médico sobre este outro medicamento.
2. O que precisa saber antes de começar a tomar Cabazitaxel Dr. Reddys
Não use Cabazitaxel Dr. Reddys:
- se é alérgico (hipersensível) a cabazitaxel, a outros taxanos, ao polisorbato 80 ou a algum dos outros excipientes deste medicamento (incluídos na secção 6),
- se o número dos seus glóbulos brancos é muito baixo (número de neutrófilos menor ou igual a 1.500/mm3),
- se tem problemas graves de fígado,
- se recentemente foi ou vai ser vacinado contra a febre amarela.
Não deve receber este medicamento se lhe suceder alguma das circunstâncias anteriores. Se não tiver certeza, consulte o seu médico antes de receber este medicamento.
Advertências e precauções
Antes de iniciar o tratamento com este medicamento, farão análises de sangue para comprovar que tem suficientes células sanguíneas e que os seus rins e fígado funcionam adequadamente para receber este medicamento.
Informar o seu médico imediatamente se:
- tiver febre. Durante o tratamento com este medicamento é mais provável que se reduza o número dos seus glóbulos brancos. O médico controlará a sua sangue e o seu estado geral para detectar sinais de infecções. Poderia administrar-lhe outros medicamentos para manter o número das suas células sanguíneas. As pessoas com recuentos celulares baixos podem desenvolver infecções que podem pôr em perigo a vida. O primeiro sinal de infecção poderia ser febre, por isso, se tiver febre, informe o seu médico imediatamente.
- alguma vez teve alguma alergia. Durante o tratamento com este medicamento podem produzir-se reacções alérgicas graves.
- tiver diarreia grave ou duradoura, se sentir mal (náuseas) ou está mal (vómitos). Qualquer uma dessas situações pode produzir desidratação grave. O seu médico teria que pôr-lhe um tratamento.
- tiver sensação de insensibilidade, formigueiro, ardor ou diminuição das sensações em mãos e pés.
- tiver algum problema de sangramento no intestino ou tem alterações na cor das suas fezes ou dor de estômago. Se o sangramento ou a dor for grave, o seu médico interromperá o seu tratamento com este medicamento. Isto é porque este medicamento poderia aumentar o risco de sangramento ou desenvolvimento de perfurações na parede intestinal.
- tiver problemas de rim.
- aparecem problemas de fígado durante o tratamento.
- tem tonalidade amarelada da pele e dos olhos, urina escura, náuseas severas (sensação de doença) ou vómitos, pois poderiam ser sinais ou sintomas de problemas hepáticos.
- nota que o volume da sua urina aumenta ou diminui significativamente.
- tiver sangue na sua urina.
Se lhe suceder alguma das circunstâncias anteriores, informe o seu médico imediatamente. O seu médico poderia reduzir a dose deste medicamento ou interromper o tratamento.
Uso de Cabazitaxel Dr. Reddys com outros medicamentos
Informar o seu médico, farmacêutico ou enfermeira se está a utilizar ou utilizou recentemente outros medicamentos, mesmo os adquiridos sem receita. Isto é devido a que alguns medicamentos podem afetar a eficácia deste medicamento ou este medicamento pode afetar a eficácia de outros medicamentos.
Estes medicamentos incluem os seguintes:
- ketoconazol, rifampicina (para infecções);
- carbamazepina, fenobarbital ou fenitoína (para convulsões);
- Erva de São João ou hipérico (Hypericum perforatum) (planta medicinal utilizada para tratar a depressão e outros problemas);
- estatinas (tais como simvastatina, lovastatina, atorvastatina, rosuvastatina, ou pravastatina) (para reduzir o colesterol no seu sangue);
- valsartan (para a hipertensão);
- repaglinida (para a diabetes).
Enquanto estiver em tratamento com este medicamento, consulte com o seu médico antes de se vacinar.
Gravidez, lactação e fertilidade
Cabazitaxel não é indicado em mulheres.
Este medicamento não deve ser utilizado em mulheres grávidas ou em idade fértil que não utilizem anticoncepcionais.
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Este medicamento não deve ser utilizado durante a lactação.
Use preservativos nas suas relações sexuais se a sua parceira está ou pode estar grávida. Este medicamento poderia estar presente no seu sêmen e pode afetar o feto. Recomenda-se não gerar um filho durante e até 6 meses após o tratamento e solicitar informações sobre a conservação do esperma antes do tratamento, pois este medicamento poderia alterar a fertilidade masculina.
Condução e uso de máquinas
Durante o tratamento com este medicamento poderia sentir-se cansado ou mareado. Se isto acontecer, não conduza nem use ferramentas ou máquinas até que se sinta melhor.
Cabazitaxel Dr. Reddys contém etanol (álcool)
Este medicamento contém 573,3 mg de álcool (etanol) em cada frasco de solvente. A quantidade na dose deste medicamento é equivalente a menos de 15 ml de cerveja ou 6 ml de vinho. A pequena quantidade de álcool neste medicamento não terá efeitos notáveis. Se é viciado em álcool, tem uma doença hepática ou epilepsia, consulte o seu médico ou farmacêutico antes de tomar este medicamento.
3. Como usar Cabazitaxel Dr. Reddys
Instruções de uso
Antes de receber este medicamento, serão administrados medicamentos antialérgicos para reduzir o risco de reacções alérgicas.
- Este medicamento será administrado por um médico ou uma enfermeira.
- Este medicamento deve ser preparado (diluído) antes de ser administrado. Com este prospecto é fornecida informação prática para a manipulação e administração deste medicamento para médicos, enfermeiras e farmacêuticos.
- Este medicamento será administrado no hospital mediante um gotejamento (perfusão) numa das suas veias (via intravenosa) durante aproximadamente 1 hora.
- Como parte do seu tratamento, tomará também um medicamento corticosteroide (prednisona ou prednisolona) por via oral todos os dias.
Quantos e com que frequência se administra
- A dose habitual depende da sua área de superfície corporal. O seu médico calculará a sua área de superfície corporal em metros quadrados (m2) e decidirá a dose que deve receber.
- Normalmente receberá uma perfusão cada 3 semanas.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeira.
Em caso de sobredose ou ingestão acidental, consulte o seu médico ou farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone: 91 562 04 20, indicando o medicamento e a quantidade ingerida.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram. O seu médico comentará isto consigo e explicará os riscos e os benefícios potenciais do seu tratamento.
Dirija-se imediatamente ao médico se notar algum dos seguintes efeitos adversos:
- febre (temperatura alta). Isto é muito frequente (pode afetar mais de 1 em cada 10 pessoas).
- perda grave de fluidos corporais (desidratação). Isto é frequente (pode afetar até 1 em cada 10 pessoas). Isto pode ocorrer se tiver diarreia grave ou duradoura, ou febre, ou se tem estado a vomitar.
- dor de estômago grave ou dor de estômago que não se resolve. Isto pode acontecer se tiver uma perfuração no estômago, esófago, intestino (perfuração gastrointestinal). Isto pode causar a morte.
Se lhe suceder alguma das circunstâncias anteriores, comunique-o imediatamente ao seu médico.
Outros efeitos adversos incluem:
Muito frequentes(podem afetar mais de 1 em cada 10 pessoas):
- redução do número de células sanguíneas vermelhas (anemia), ou brancas (que são importantes para combater as infecções)
- redução do número de plaquetas (o que resulta num aumento do risco de ter hemorragias)
- perda de apetite (anorexia)
- alteração do gosto
- respiração entrecortada
- tosse
- molestias de estômago, incluindo náuseas, vómitos, diarreia ou prisão de ventre
- dor abdominal
- perda de cabelo a curto prazo (na maioria dos casos o cabelo volta a crescer com normalidade)
- dor de costas
- dor das articulações
- sangue na urina
- cansaço, fraqueza ou falta de energia.
Frequentes(podem afetar até 1 em cada 10 pessoas):
- alteração do gosto
- dificuldade para respirar
- tosse
- dor abdominal
- perda de cabelo a curto prazo (na maioria dos casos, deve produzir-se o crescimento normal do cabelo)
- dor nas articulações
- infecção do trato urinário
- escassez de glóbulos brancos associada com febre e infecções
- sensação de insensibilidade, formigueiro, ardor ou diminuição das sensações em mãos e pés
- tontura
- dor de cabeça
- aumento ou diminuição da tensão arterial
- malestar de estômago, ardor de estômago ou arrotos
- dor de estômago
- hemorroides
- espasmos musculares
- urinar com frequência ou com dor
- incontinência urinária
- problemas ou alteração dos rins
- úlceras na boca ou nos lábios
- infecções ou risco de infecções
- nível de açúcar no sangue elevado
- insónia
- confusão mental
- sensação de ansiedade
- sensação rara ou perda de sensação ou dor em mãos e pés
- zumbidos nos ouvidos
- problemas de equilíbrio
- batimentos rápidos ou irregulares do coração
- coágulos de sangue nas pernas ou nos pulmões
- sensação de calor ou sofoco na pele
- dor de boca ou garganta
- hemorragia retal
- pele avermelhada
- molestias, distúrbios, fraqueza ou dores musculares
- inflamação de pés ou pernas
- arrepios
- distúrbios das unhas (mudança na cor das unhas; as unhas podem desprender-se).
Pouco frequentes(podem afetar até 1 em cada 100 pessoas):
- níveis baixos de potássio no sangue
- zumbidos no ouvido
- calor na pele
- avermelhamento da pele.
- inflamação da bexiga, que pode ocorrer quando a sua bexiga esteve previamente exposta a radioterapia (cistite devido a fenómenos de recorde de radiação).
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis)
- doença pulmonar intersticial (inflamação dos pulmões causando tosse e dificuldade para respirar).
Comunicação de efeitos adversos:
Se experimentar algum tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Cabazitaxel Dr. Reddys
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no envase e na etiqueta dos frascos após CAD. A data de validade é o último dia do mês que se indica.
Não refrigerar.
Na secção “INFORMAÇÃO PRÁTICA PARA MÉDICOS OU PROFISSIONAIS DE SAÚDE SOBRE A PREPARAÇÃO, ADMINISTRAÇÃO E MANIPULAÇÃO DE CABAZITAXEL DR. REDDYS” é incluída informação sobre a conservação e o tempo de uso deste medicamento, uma vez que se encontra diluído e está pronto para usar.
A eliminação do medicamento não utilizado e de todos os materiais que estiveram em contacto com ele, será realizada de acordo com a normativa local. Estas medidas ajudarão a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Cabazitaxel Dr. Reddys
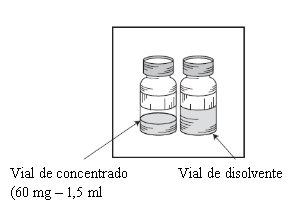
O princípio ativo é cabazitaxel. Um ml de concentrado contém 40 mg de cabazitaxel. Cada frasco de concentrado contém 60 mg de cabazitaxel.
Os outros componentes são polissorbato 80 e ácido cítrico no concentrado, e etanol 96% e água para preparações injetáveis no solvente (ver seção 2 “Cabazitaxel Dr. Reddys contém etanol (álcool)”).
Nota: tanto o frasco do concentrado de Cabazitaxel Dr. Reddys 60 mg/1,5 ml (volume de enchimento: 73,2 mg de cabazitaxel/1,83 ml) como o frasco de solvente (volume de enchimento: 5,70 ml) contêm um sobre-enchimento para compensar a perda de líquido durante a preparação. Este sobre-enchimento assegura que após a diluição com o conteúdo COMPLETOdo solvente fornecido, haja uma solução contendo 10 mg/ml de cabazitaxel.
Aspecto do produto e conteúdo do envase
Cabazitaxel Dr. Reddys é um concentrado e solvente para solução para perfusão (concentrado estéril).
O concentrado é uma solução oleosa transparente, de cor amarela a amarelada-marronácea.
O solvente é uma solução transparente e incolor.
Um envase de Cabazitaxel Dr. Reddys contém:
- Um frasco de uso único de vidro transparente, fechado com um tampão de borracha clorobutilo de cor laranja, selado com uma cápsula de alumínio, coberto com um tampão expulsor flip-off de plástico de cor verde claro, contendo 1,5 ml (volume nominal) de concentrado.
- Um frasco de uso único de vidro transparente, fechado com um tampão de borracha clorobutilo de cor cinza, selado com uma cápsula de alumínio dourada, coberto com um tampão expulsor flip-off de plástico incolor, contendo 4,5 ml (volume nominal) de solvente.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização
Reddy Pharma Iberia, S.A.
Avda. Josep Tarradellas nº 38
08029 Barcelona (Espanha)
Nº Telefone: 93.355.49.16
Nº Fax: 93.355.49.61
Responsável pela fabricação
betapharm Arzneimittel GmbH,
Kobelweg 95,
86156 Augsburg
Alemanha
Este medicamento está autorizado nos Estados-Membros do Espaço Económico Europeu com os seguintes nomes:
País | Nome proposto |
DE | Cabazitaxel beta 60 mg Konzentrat und Lösungsmittel zur Herstellung einer Infusionslösung |
ES | Cabacitaxel Dr. Reddys 60 mg concentrado y disolvente para solución para perfusión EFG |
FR | CABAZITAXEL REDDY PHARMA 60 mg, solution à diluer et solvant pour solution pour perfusion |
IT | Cabazitaxel Dr. Reddy´s |
UK | Cabazitaxel 60 mg Concentrate And Solvent For Solution For Infusion |
Data da última revisão deste prospecto:Abril 2024
A seguinte informação está destinada apenas a profissionais de saúde.
INFORMAÇÃO PRÁTICA PARA MÉDICOS OU PROFISSIONAIS DE SAÚDE SOBRE
A PREPARAÇÃO, ADMINISTRAÇÃO E MANIPULAÇÃO DE CABAZITAXEL DR. REDDYS 60 mg
CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Esta informação complementa as seções 3 e 5 para o usuário.
É importante que leia o conteúdo completo deste procedimento antes de preparar a solução para perfusão.
Incompatibilidades
Este medicamento não deve ser misturado com outros medicamentos, exceto os utilizados para as diluições.
Não devem ser usados recipientes de infusão de PVC ou conjuntos de infusão de poliuretano para a preparação e administração da solução para infusão.
Período de validade e precauções especiais de conservação
Para o envase de Cabazitaxel Dr. Reddys 60 mg concentrado e solvente
Não refrigerar.
Após a abertura do frasco
Os frascos de concentrado e solvente devem ser usados imediatamente. Se não forem usados imediatamente, o tempo e as condições de conservação são responsabilidade do usuário. Desde um ponto de vista microbiológico, o processo de diluição em duas etapas deve ser realizado em condições controladas e assépticas (ver a seguir “Precauções de preparação e administração”).
Após a diluição inicialde Cabazitaxel Dr. Reddys 60 mg concentrado com o conteúdo COMPLETOdo frasco de solvente: demonstrou-se a estabilidade química e física em uso durante 1 hora a temperatura ambiente e durante 21 dias a 2ºC - 8ºC.
Após a diluição final na bolsa/botija de perfusão
Demonstrou-se a estabilidade química e física da solução de perfusão durante 8 horas a temperatura ambiente (15ºC - 30ºC) incluindo 1 hora de tempo de perfusão e durante 48 horas na geladeira incluindo a hora de tempo de perfusão.
Desde um ponto de vista microbiológico, a solução de perfusão deve ser usada imediatamente. Se não for usada imediatamente, os tempos e as condições de conservação são responsabilidade do usuário e normalmente não devem ser mais de 24 horas a 2ºC - 8ºC, a menos que a diluição tenha sido realizada em condições assépticas controladas e validadas.
Precauções de preparação e administração
Assim como outros agentes antineoplásicos, deve-se agir com precaução durante a preparação e administração das soluções de Cabazitaxel Dr. Reddys, tendo em conta o uso de dispositivos de segurança, equipamento de proteção pessoal (por exemplo, luvas) e procedimentos de preparação.
Se em qualquer uma das etapas de preparação, Cabazitaxel Dr. Reddys entrar em contato com a pele, lavar imediatamente e minuciosamente com água e sabão. Se entrar em contato com membranas mucosas, lavar imediatamente e minuciosamente com água.
Cabazitaxel Dr. Reddys só deve ser preparado e administrado por pessoal treinado no manejo de agentes citotóxicos. As trabalhadoras grávidas não devem manipulá-lo.
Diluir sempre o concentrado para solução para perfusão com o solvente COMPLETOque se fornece antes de adicioná-lo às soluções de perfusão.
Etapas da preparação
Leia atentamente TODAesta seção antes de misturar e diluir. Cabazitaxel Dr. Reddys requer DUASdiluições antes da administração. Siga as instruções de preparação que se fornecem a seguir.
Nota: tanto o frasco do concentrado de Cabazitaxel Dr. Reddys 60 mg/1,5 ml (volume de enchimento: 73,2 mg de cabazitaxel/1,83 ml) como o frasco de solvente (volume de enchimento: 5,67 ml) contêm um sobre-enchimento para compensar a perda de líquido durante a preparação. Este sobre-enchimento assegura que após a diluição com o conteúdo COMPLETOdo solvente fornecido, haja uma solução contendo 10 mg/ml de cabazitaxel.
Para preparar a solução para perfusão, o seguinte processo de diluição em duas etapas deve ser realizado de forma asséptica.
Etapa 1: diluição inicial do concentrado de solução para perfusão com o solvente fornecido.
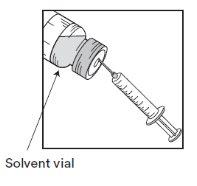
Etapa 1.1
Inspeccionar o frasco de concentrado cierre flip-off laranja e o solvente fornecido. A solução de concentrado e de solvente devem ser transparentes. |
|
Etapa 1.2
Utilizando uma seringa provida de uma agulha fixa, extrair de forma asséptica o conteúdo COMPLETOdo solvente fornecido invertendo parcialmente o frasco. |
|
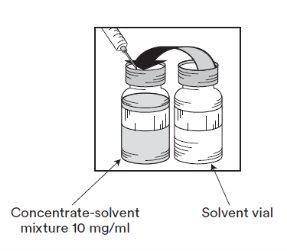
Etapa 1.3
Injetar o conteúdo COMPLETOno correspondente frasco de concentrado cierre flip-off laranja. Para limitar todo o possível a formação de espuma ao injetar o solvente, dirigir a agulha para a parede interior do frasco de solução de concentrado e injetar lentamente. Uma vez reconstituído, a solução resultante contém 10 mg/ml de cabazitaxel. |
|
Etapa 1.4
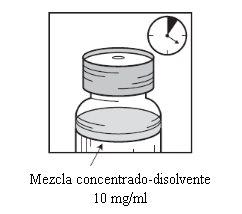
Sacar a seringa e a agulha e misturar manualmente e suavemente, mediante inversões repetidas, até que se obtenha uma solução transparente e homogênea. Podem ser necessários uns 45 segundos. |
|
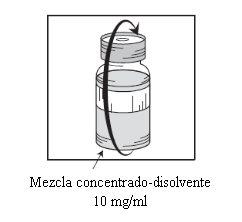
Etapa 1.5
Deixar repousar a solução durante aproximadamente 5 minutos e a seguir verificar que a solução é homogênea e transparente. É normal que persista a espuma passado este tempo. |
|
Esta mistura concentrado-solvente resultante contém 10 mg/ml de cabazitaxel (pelo menos 6 ml de volume liberado). A segunda diluição deve ser realizada imediatamente (antes de 1 hora) como se detalha na Etapa 2.
Pode ser necessário mais de um frasco de mistura concentrado-solvente para administrar a dosagem prescrita.
Etapa 2: segunda diluição (final) para perfusão
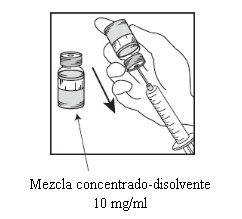
Etapa 2.1
De forma asséptica extrair a quantidade necessária de mistura concentrado-solvente (10 mg/ml de cabazitaxel), com uma seringa graduada provida de uma agulha fixa. Como exemplo, uma dosagem de 45 mg de Cabazitaxel Dr. Reddys requeriria 4,5 ml da mistura de concentrado-solvente preparada na Etapa 1. Como pode seguir havendo espuma na parede do frasco desta solução, após a preparação descrita na Etapa 1, é preferível situar a agulha da seringa na metade do conteúdo durante a extração. |
|
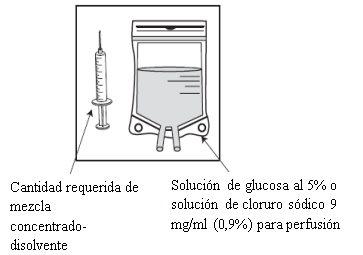
Etapa 2.2
Injetar em um envase estéril sem PVC de solução de glicose a 5% ou solução de cloreto de sódio 9 mg/ml (0,9%) para perfusão. A concentração da solução para perfusão deve estar entre 0,10 mg/ml e 0,26 mg/ml. |
|
Etapa 2.3
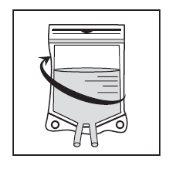
Sacar a seringa e misturar o conteúdo da bolsa ou botija de perfusão manualmente, mediante movimento de balanço. |
|
Etapa 2.4
Assim como todos os produtos parenterais, a solução de perfusão resultante deve ser inspecionada visualmente antes de usá-la. Como a solução de perfusão está sobressaturada, pode cristalizar com o tempo. Neste caso, não se deve usar a solução e deve ser eliminada. |
|
A solução para perfusão deve ser usada imediatamente. No entanto, o tempo de conservação em uso pode ser mais longo sob as condições específicas mencionadas na seção Período devalidade e precauções especiais de conservação.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele, será realizada de acordo com a normativa local.
Método de administração
Cabazitaxel Dr. Reddys é administrado em perfusão durante 1 hora.
Recomenda-se o uso de um filtro em linha de 0,22 micrómetros de tamanho de poro nominal (também denominado 0,2 micrómetros) durante a administração.
Não devem ser usados envases de perfusão de PVC ou conjuntos de perfusão de poliuretano para a preparação e administração da solução para perfusão.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃOForma farmacêutica: PERFURAÇÃO INJETÁVEL, 20 mg/mlSubstância ativa: cabazitaxelFabricante: Accord Healthcare S.L.U.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 20 mgSubstância ativa: cabazitaxelFabricante: Eugia Pharma (Malta) LimitedRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 10 mg/mlSubstância ativa: cabazitaxelFabricante: Ever Valinject GmbhRequer receita médica
Alternativas a CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO em Polónia
Alternativa a CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO em Ukraine
Médicos online para CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de CABAZITAXEL DR. REDDYS 60 mg CONCENTRADO E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.