
BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO

Como usar BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO
Introdução
RESUMO DAS CARACTERÍSTICAS DO PRODUTO: INFORMAÇÃO PARA O UTILIZADOR
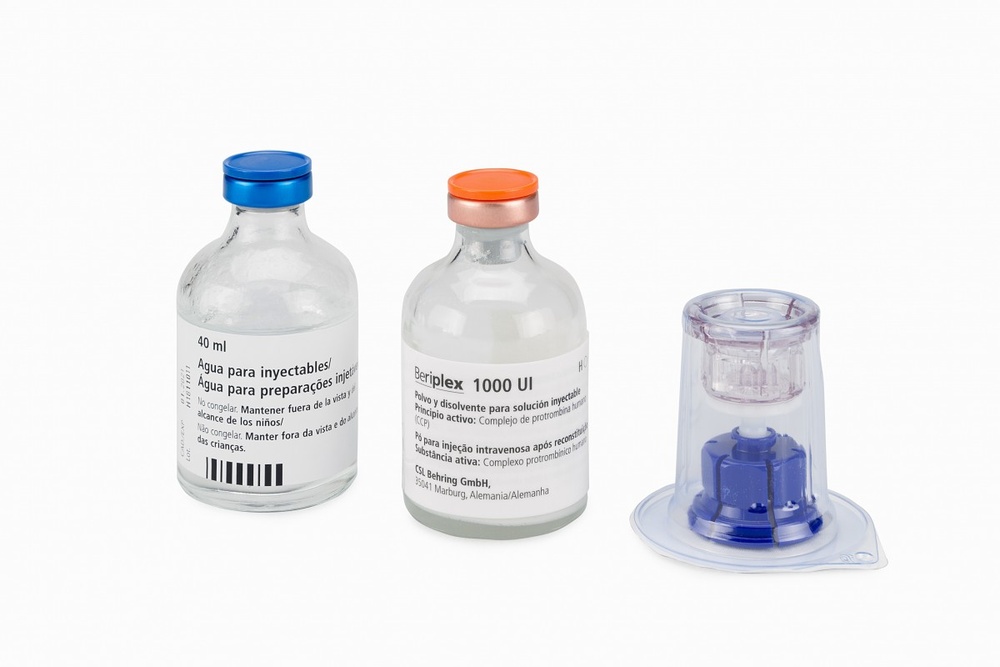
Beriate 1000 UI pó e diluente para solução injetável e para perfusão
Fator VIII de coagulação humano
Leia todo o resumo das características do produto atentamente antes de começar a usar este medicamento porque contém informações importantes para si.
- Conserva este resumo das características do produto, pois pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi prescrito apenas para si e não deve ser dado a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste resumo das características do produto. Ver seção 4.
Conteúdo do resumo das características do produto:
- O que é Beriate e para que é utilizado
- O que precisa saber antes de começar a usar Beriate
- Como usar Beriate
- Posíveis efeitos adversos
- Conservação de Beriate
- Conteúdo do envase e informações adicionais
1. O que é Beriate e para que é utilizado
O que é Beriate
Beriate apresenta-se como pó, acompanhado do diluente. A solução obtida deve ser administrada numa veia, bem por injeção, bem por perfusão.
Beriate é obtido do plasma humano (que é a parte líquida do sangue) e contém o fator VIII de coagulação humano. É utilizado para prevenir ou deter as hemorragias que são causadas pela falta do fator VIII (hemofilia A) no sangue. Pode ser utilizado também no tratamento da deficiência adquirida de fator VIII.
Para que é utilizado Beriate
O fator VIII está envolvido na coagulação do sangue. A falta de fator VIII significa que o sangue não coagula com a rapidez que deveria fazer, com o que se produz um aumento da tendência ao sangramento. Beriate fornece fator VIII, com o que se normalizam, temporariamente, os mecanismos de coagulação.
2. O que precisa saber antes de começar a usar Beriate
Os seguintes parágrafos contêm informações que si e o seu médico devem ter em consideração antes de usar Beriate.
Não use Beriate:
- Se é alérgico ao fator VIII de coagulação humano ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6).
Advertências e precauções
Rastreabilidade
Recomenda-se encarecidamente que cada vez que se administre Beriate, registe a data de administração, o número do lote e o volume injetado no seu diário de tratamento.
Consulte o seu médico ou farmacêutico antes de começar a usar Beriate
- É possível que se apresentem reações de hipersensibilidade de tipo alérgico. O seu médico deverá informá-lo sobre a ocorrência de sintomas precoces de reações de hipersensibilidade, incluindo urticárias, erupções cutâneas generalizadas, pressão no peito, respiração dificultosa, queda da tensão sanguínea e anafilaxia (reação alérgica grave que causa problemas graves na respiração, ou tonturas). Se se apresentarem estes sintomas, deve interromper imediatamente a administração do medicamento e contactar o seu médico.
- A formação de inibidores(anticorpos) é uma complicação conhecida que pode ocorrer durante o tratamento com todos os medicamentos compostos por fator VIII. Estes inibidores, especialmente em grandes quantidades, impedem que o tratamento funcione corretamente, por isso serão supervisionados cuidadosamente si e o seu filho por si desenvolvem ditos inibidores. Se a hemorragia ou a do seu filho não se está controlando com Beriate, consulte o seu médico imediatamente.
- Se padece uma doença cardíaca ou tem risco de padecer, informe o seu médico ou farmacêutico.
- Se para a administração de Beriate for necessário um dispositivo de acesso venoso central (DAVC) o seu médico deverá considerar o risco de complicações relacionadas com o catéter, incluindo as infecções locais, bacterianas (bacteriemia) e a formação de coágulos de sangue (trombose) no local de inserção do catéter.
O seu médico avaliará cuidadosamente o benefício do tratamento com Beriate face ao risco destas complicações.
Segurança viral
Quando se administram medicamentos derivados do sangue ou plasma humanos, adoptam-se certas medidas para prevenir a transmissão de infecções aos pacientes. Isso inclui a seleção cuidadosa dos doadores de sangue e plasma para excluir aqueles que podem representar um risco de transmissão de infecções, e a análise de cada doador e bancos de plasma para sinais de vírus/infecções. Os fabricantes destes produtos também incluem etapas nos processos de produção que podem inativar ou eliminar vírus ou outros patógenos. Apesar disso, quando se administram medicamentos derivados do sangue ou plasma humanos, a possibilidade de transmissão de agentes infecciosos não pode ser excluída totalmente. Isso também se refere a vírus emergentes ou de natureza desconhecida e outros patógenos.
Estes procedimentos são considerados eficazes face a vírus envoltos, tais como o vírus da imunodeficiência humana (VIH, vírus da SIDA), o vírus da hepatite B e o vírus da hepatite C (inflamação do fígado), assim como para vírus não envoltos, tais como o vírus da hepatite A e parvovirus B19.
O seu médico pode recomendar que considere a vacinação contra a hepatite A e B se regularmente/repetidamente se lhe administram produtos derivados de plasma humano (por exemplo, fator VIII).
Uso de Beriate com outros medicamentos:
- Comunique ao seu médico ou farmacêutico se está utilizando, utilizou recentemente ou poderia ter que utilizar qualquer outro medicamento.
- Beriate não deve ser misturado com outros medicamentos, diluentes e solventes, excepto aqueles que são recomendados pelo fabricante (ver seção 6).
Gravidez, lactação e fertilidade
- Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
- Durante a gravidez e a lactação, Beriate só deve ser utilizado no caso de que esteja claramente indicado.
- Não se dispõe de informações sobre fertilidade
Condução e uso de máquinas
Beriate não afeta a capacidade de conduzir veículos ou utilizar maquinaria.
Beriatecontém sódio
Beriate 1000 UI contém 27,55 mg de sódio (componente principal do sal de cozinha) em cada frasco. Isso equivale a 1,4% da ingestão máxima diária de sódio recomendada para um adulto.
3. Como usar Beriate
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
O tratamento da hemofilia A deve ser iniciado sob a supervisão de um médico com experiência no tratamento deste tipo de alterações.
Posologia
A dose de fator VIII que precisa e a duração do tratamento dependem de vários fatores, tais como o seu peso corporal, a gravidade da sua doença, a localização e importância da hemorragia ou a necessidade de prevenir a hemorragia durante uma operação ou uma revisão médica.
Se lhe foi indicado que deve usar Beriate em casa, o seu médico se certificará de que recebe as instruções devidas sobre como injetar o produto e quanto produto deve usar.
Siga as instruções que lhe deu o seu médico ou as indicações das enfermeiras do seu centro de hemofilia.
Uso em crianças e adolescentes
A dose é calculada com base no peso corporal e é determinada da mesma maneira que para os adultos.
Se usar mais Beriate do que deve
Não foram relatados sintomas de sobredosagem com o fator VIII.
Se esquecer de usar Beriate
Aplique imediatamente a próxima dose e continue a intervalos regulares, seguindo as instruções do seu médico. Não tome uma dose dupla para compensar as doses esquecidas.
Reconstituição e administração
Instruções gerais:
- O pó deve ser misturado (reconstituído) com o solvente (líquido) e retirado do frasco sob condições assépticas.
- A solução reconstituída deve ser clara ou ligeiramente opalescente, ou seja, pode brilhar quando se sobrepõe contra uma luz. Ocasionalmente, podem aparecer alguns flocos ou partículas no frasco. O filtro incluído no Mix2Vial elimina estas partículas. Esta filtração não afeta os cálculos das doses. Depois da filtração e da transferência do produto reconstituído para a seringa (ver mais adiante), e antes da administração, a solução deve ser controlada mediante um controle visual para detectar pequenas partículas e decolorações. Não use as soluções que apresentem turbidez visível ou que contenham flocos ou partículas na seringa.
- Uma vez que o produto é transferido para a seringa, deve ser usado imediatamente. Nãoarmazene o produto na seringa.
- O produto não utilizado e os materiais descartados devem ser eliminados adequadamente de acordo com os requisitos locais e de acordo com as instruções do seu médico.
Reconstituição:
Aqueça os frascos de Beriate (frasco com o pó e o frasco com o líquido), sem abri-los, a temperatura ambiente. Isso pode ser feito deixando os frascos a temperatura ambiente durante 1 hora, ou mantendo-os nas mãos durante alguns minutos. NÃO exponha os frascos ao calor direto. Os frascos não devem ser aquecidos acima da temperatura corporal (37 ºC).
Retire cuidadosamente as cápsulas protetoras dos frascos que contêm o pó e o solvente, e limpe a parte exposta dos tampões com um pano embebido em álcool. Deixe secar os frascos antes de abrir o contenedor do Mix2Vial, e siga as seguintes instruções.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aplicação e retirada:
|
|
9 |
|
Use o equipamento de punção venosa que é fornecido com o produto. Insira a agulha na veia. Deixe que o sangue entre até o final do tubo. Conecte a seringa ao final rosqueado e com fechamento do equipamento de punção venosa. Injete lentamente a solução reconstituída na veia,seguindo as instruções do seu médico. A velocidade de injeção ou de perfusão não deve ultrapassar 2 ml por minuto. Tenha cuidado para que não entre sangue na seringa que contém o produto.
Quando deva ser administrado um volume grande, a perfusão é uma opção a considerar. O produto reconstituído deve ser transferido para um sistema de perfusão autorizado. A perfusão deve ser realizada de acordo com as instruções do seu médico.
Verifique-se sobre qualquer efeito adverso que possa ocorrer imediatamente. Se você tiver algum efeito adverso relacionado à administração de Beriate, a injeção ou a perfusão deve ser interrompida (ver também seção 2).
Se tiver alguma outra pergunta sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Posíveis efeitos adversos
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Se algum dos seguintes efeitos adversos ocorrer, consulte o seu médico imediatamente ou vá ao Serviço de Urgência ou centro de hemofilia no seu hospital mais próximo:
- Sintomas de angioedema
- inflamação da face, língua ou faringe,
- dificuldade para engolir,
- urticárias e dificuldade para respirar.
Estes efeitos adversos foram observados muito raramente (podem afetar até 1 de cada 10.000 pessoas), e em alguns casos podem dar origem a reações alérgicas graves (anafilaxia), incluindo o choque
- Perda de efeito (sangramento não se detém). Nos crianças que não receberam tratamento prévio com medicamentos compostos por fator VIII podem ocorrer anticorpos inibidores (ver seção 2) muito frequentemente (mais de 1 de cada 10 pacientes); no entanto, nos pacientes que receberam tratamento prévio com fator VIII (mais de 150 dias de tratamento), o risco é pouco frequente (menos de 1 de cada 100 pacientes). Se isso ocorrer, os medicamentos que você ou seu filho tomam podem deixar de funcionar corretamente e você ou seu filho podem sofrer uma hemorragia persistente. Nesse caso, contate o seu médico imediatamente.
Outros efeitos adversos são:
- Reações alérgicas (hipersensibilidade), que podem incluir:
- sensação de queimadura e picada no local onde se injetou ou perfundiu o produto.
- calafrios, rubor, erupção cutânea de todo o corpo, pápulas.
- dor de cabeça.
- queda da pressão sanguínea, inquietude, batida do coração mais rápida, opressão no peito, respiração dificultosa.
- sonolência (letargia).
- náuseas, vômitos.
- formigamento.
Estes efeitos adversos foram observados muito raramente, mas em alguns casos podem evoluir para reações alérgicas graves (anafilaxia), incluindo o choque.
- Em muito raras ocasiões foi observada febre.
Efeitos adversos em crianças e adolescentes
A frequência, tipo e gravidade das reações adversas em crianças se espera que sejam as mesmas que para adultos.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste resumo das características do produto. Também pode comunicá-los diretamente através do Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Beriate
Não use este medicamento após a data de validade que aparece na etiqueta e no envase. A data de validade é o último dia do mês que se indica.
- Conservar na geladeira (entre 2 ºC e 8 °C).
- Durante o seu período de validade, Beriate pode ser armazenado até 25 ºC, durante um período de 1 mês como máximo. Este período de armazenamento a temperatura ambiente deve ser anotado no seu diário de tratamento para cumprir o período de 1 mês em sua totalidade.
- Beriate não contém conservantes, por isso o produto reconstituído deve ser preferivelmente usado imediatamente.
- Se o produto reconstituído não for administrado imediatamente, o armazenamento no frasco a temperatura ambiente não deve exceder um período de 8 horas. Uma vez transferido para a seringa, o produto deve ser usado imediatamente.
- Não congelar.
- Conservar o frasco no embalagem original para protegê-lo da luz.
Mantenha fora da vista e do alcance das crianças.
6. Conteúdo do envase e informação adicional
Composição de Beriate
O princípio ativo é:
Beriate apresenta-se como um pó (contendo nominalmente 1000 UI de fator VIII de coagulação humano por frasco) e um líquido (dissolvente). A solução reconstituída é administrada, seja por injeção, seja por perfusão.
Beriate 1000 UI é reconstituído com 10 ml de água para preparações injetáveis e contém aproximadamente 100 UI/ml de fator VIII de coagulação humano.
Os demais componentes são:
Glicina, cloreto de cálcio, hidróxido de sódio (em pequenas quantidades) para ajustar o pH, sacarose e cloreto de sódio
Dissolvente: água para preparações injetáveis 10 ml.
Aspecto do produto e conteúdo do envase
Beriate apresenta-se como um pó branco, e é fornecido com a correspondente água para preparações injetáveis.
A solução reconstituída deve ser clara ou ligeiramente opalescente, ou seja, pode brilhar quando sobreposta contra uma luz, mas não deve conter partículas.
Apresentações:
Caixa com 1000 UI que contém:
- 1 frasco com pó
- 1 frasco com 10 ml de Água para preparações injetáveis
- 1 trasvasador com filtro 20/20
Equipamento de administração (caixa interior):
- 1 seringa de 10 ml de uso único
- 1 equipamento de venopunção
- 2 toalhetas impregnadas de álcool
- 1 penso não estéril
Titular da Autorização de comercialização e responsável pela fabricação
CSL Behring GmbH
Emil-von-Behring-Straße 76
35041 Marburg, Alemanha
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
CSL Behring, S.A.
c/ Tarragona 157, planta 18
08014 Barcelona
Espanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Áustria:
Beriate 100 I.E./ml Pó e dissolvente para solução injetável ou infusão (250 I.E., 500 I.E., 1000 I.E.)
Beriate 200 I.E./ml Pó e dissolvente para solução injetável ou infusão (2000 I.E.)
Bulgária:
Beriate 250 IU Pó e dissolvente para solução injetável ou infusão
Beriate 500 IU Pó e dissolvente para solução injetável ou infusão
Beriate 1000 IU Pó e dissolvente para solução injetável ou infusão
Beriate 2000 IU Pó e dissolvente para solução injetável ou infusão
Croácia:
Beriate 250 IU pó e solvente para solução injetável ou infusão
Beriate 500 IU pó e solvente para solução injetável ou infusão
Beriate 1000 IU pó e solvente para solução injetável ou infusão
Beriate 2000 IU pó e solvente para solução injetável ou infusão
República Checa:
Beriate 250 IU, Beriate 500 IU, Beriate 1000 IU, Beriate 2000 IU
Estônia:
Beriate
Alemanha:
Beriate 250, Beriate 500, Beriate 1000, Beriate 2000
Hungria:
Apresentações de Beriate 250, 500 e 1000:
BERIATE 100 NE/ml pó e solvente para solução injetável ou infusão
Apresentação 2000:
BERIATE 200 NE/ml pó e solvente para solução injetável ou infusão
Itália:
Beriate
Letônia:
Beriate 250 SV pó e solvente para solução injetável ou infusão
Beriate 500 SV pó e solvente para solução injetável ou infusão
Beriate 1000 SV pó e solvente para solução injetável ou infusão
Beriate 2000 SV pó e solvente para solução injetável ou infusão
Lituânia:
Beriate® 250 TV pó e solvente para solução injetável ou infusão
Beriate® 500 TV pó e solvente para solução injetável ou infusão
Beriate® 1000 TV pó e solvente para solução injetável ou infusão
Beriate® 2000 TV pó e solvente para solução injetável ou infusão
Polônia:
Beriate 250
Beriate 500
Beriate 1000
Beriate 2000
Portugal:
Beriate
Romênia:
Beriate 250 pó e solvente para solução injetável ou infusão
Beriate 500 pó e solvente para solução injetável ou infusão
Beriate 1000 pó e solvente para solução injetável ou infusão
Beriate 2000 pó e solvente para solução injetável ou infusão
Espanha:
Beriate 500 UI pó e dissolvente para solução injetável e para perfusão
Beriate 1000 UI pó e dissolvente para solução injetável e para perfusão
Beriate 2000 UI pó e dissolvente para solução injetável e para perfusão
Eslováquia:
Beriate 250 IU
Beriate 500 IU
Beriate 1000 IU
Beriate 2000 IU
Eslovênia:
Beriate 250 i.e. pó e veículo para solução injetável ou infusão
Beriate 500 i.e. pó e veículo para solução injetável ou infusão
Beriate 1000 i.e. pó e veículo para solução injetável ou infusão
Beriate 2000 i.e. pó e veículo para solução injetável ou infusão
Data da última revisão deste prospecto:Abril 2020
A informação detalhada e atualizada sobre este medicamento está disponível no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS)http://www.aemps.gob.es.
----------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais do setor sanitário
Posologia
Monitorização do tratamento
Durante o tratamento, recomenda-se controlar, adequadamente, os níveis de fator VIII para determinar a dose a administrar e a frequência das perfusões repetidas. A resposta de cada paciente ao fator VIII pode variar, demonstrando diferentes meias-vidas e recuperações. A dose baseada no peso corporal pode requerer um ajuste em pacientes com baixo peso ou sobrepeso. No caso particular de intervenções de cirurgia maior, é indispensável monitorizar com precisão a terapia de substituição mediante testes de coagulação (atividade plasmática do fator VIII).
Deve monitorizar-se nos pacientes o desenvolvimento de inibidores do fator VIII. Ver também seção 2.
O número de unidades de fator VIII administradas é expresso em Unidades Internacionais (UI), em relação ao padrão vigente da Organização Mundial da Saúde (OMS) para concentrados de fator VIII. A atividade plasmática de fator VIII é expressa como um percentual (em relação ao plasma humano normal) ou preferivelmente em UI (em relação a um padrão internacional para o fator VIII no plasma).
A atividade de uma UI de fator VIII é equivalente à quantidade de fator VIII contida em um ml de plasma humano normal.
Tratamento a demanda
O cálculo da dose necessária de fator VIII baseia-se na observação empírica de que 1 UI de fator VIII por kg de peso corporal incrementa a atividade plasmática de fator VIII aproximadamente em um 2% (2 UI/dL) sobre a atividade normal. A dose requerida é determinada mediante a fórmula seguinte:
Unidades requeridas = peso corporal (kg) x aumento desejado de fator VIII [% ou UI/dl] x 0,5
A quantidade administrada e a frequência de administração serão sempre estabelecidas em função da eficácia observada em cada caso.
Nos seguintes episódios hemorrágicos, a atividade de fator VIII não deve ser inferior ao nível plasmático de atividade que se indica (em % do nível normal ou UI/dL), durante o período correspondente. A tabela seguinte pode ser usada como uma guia posológica em episódios hemorrágicos e cirurgia.
Tipo de episódio hemorrágico/ Tipo de procedimento cirúrgico | Nível de fator VIII requerido (% ou UI/dL) | Frequência de dosificação (horas) / Duração da terapia (dias) |
Hemorragia | ||
Hemartrose precoce, sangramento muscular ou da cavidade bucal | 20-40 | Repetir cada 12-24 horas. Pelo menos 1 dia, até que a hemorragia se tenha resolvido, em função da dor, ou até a cicatrização adequada da ferida |
Hemartrose mais extensa, sangramento muscular ou hematoma | 30-60 | Repetir a perfusão cada 12-24 horas, durante 3-4 dias ou mais, até que a dor e a discapacidade aguda se tenham resolvido |
Hemorragias com risco vital | 60-100 | Repetir a perfusão cada 8-24 horas até que desapareça o risco |
Cirurgia | ||
Cirurgia menor, incluindo extrações dentárias | 30-60 | Cada 24 horas, pelo menos 1 dia, até a cicatrização da ferida |
Cirurgia maior | 80-100 (pré e pós-operatório) | Repetir a perfusão cada 8-24 horas até a cicatrização adequada da ferida; continuar a terapia durante um mínimo de 7 dias mais para manter uma atividade de fator VIII de 30 a 60 % (30-60 UI/dl correspondentes a 0,30-0,60 UI/ml) |
Profilaxia
Para a profilaxia a longo prazo das hemorragias em pacientes com hemofilia A grave, a dose habitual é de 20 a 40 UI de fator VIII/kg de peso corporal a intervalos de 2 a 3 dias. Em alguns casos, especialmente em pacientes jovens, pode ser necessário encurtar os intervalos de administração ou administrar doses mais elevadas.
População pediátrica
A posologia em pediatria baseia-se no peso corporal e, portanto, segue, em geral, as mesmas diretrizes que se usam para os adultos. A frequência de administração deve estar sempre orientada a conseguir a eficácia clínica em cada caso particular. Existe alguma experiência no tratamento de crianças menores de 6 anos.
Informação sobre as propriedades farmacológicas do Fator de von Willebrand
Além da função protetora do fator VIII, o fator de von Willebrand facilita a adesão das plaquetas nos sítios com uma lesão vascular e desempenha um papel na agregação plaquetária.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃOForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1500 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1000 UI - após reconstituição em 2 ml de água para injetáveis, a dose é de 500 UI/mlSubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médica
Alternativas a BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO em Polska
Alternativa a BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO em Ukraina
Médicos online para BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de BERIATE 1000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO – sujeita a avaliação médica e regras locais.




 1
1 2
2 3
3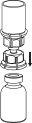 4
4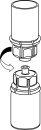 5
5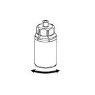 6
6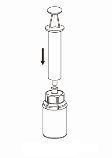 7
7 8
8









