VEDROP 50 MG/ML EN SOLUCION ORAL

Cómo usar VEDROP 50 MG/ML EN SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Vedrop 50 mg/ml en solución oral
Tocofersolán
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.Ver sección 4.
Contenido del prospecto:
- Qué es Vedrop y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Vedrop
- Cómo tomar Vedrop
- Posibles efectos adversos
- Conservación de Vedrop
- Contenido del envase e información adicional
1. Qué es Vedrop y para qué se utiliza
Vedrop contiene vitamina E (en forma de tocofersolán). Este medicamento se utiliza para tratar la carencia de vitamina E producida por la malabsorción digestiva (por la que los nutrientes de los alimentos no se absorben fácilmente durante la digestión) en pacientes con edades comprendidas entre el nacimiento (recién nacidos a término) hasta los 18 años con colestasis crónica (enfermedad hereditaria o congénita en la que la bilis no puede pasar del hígado al intestino).
2. Qué necesita saber antes de empezar a tomar Vedrop
No tome Vedrop
- Si es alérgico a la vitamina E (d-alfa tocoferol o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Vedrop no debe administrarse a bebés prematuros.
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar Vedrop si tiene:
- Problemas de riñón o deshidratación. Vedrop deberá usarse con precaución y deberá vigilarse cuidadosamente la función renal, ya que el polietilenglicol, que forma parte del principio activo tocofersolán, puede dañar los riñones.
- Problemas de hígado. Vedrop deberá usarse con precaución y las funciones del hígado deberán vigilarse estrictamente.
Toma de Vedrop con otros medicamentos
Informe a su médico o farmacéutico si está tomando o ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Informe a su médico o farmacéutico si está tomando:
- Ciertos medicamentos que disminuyen la viscosidad de la sangre (anticoagulantes orales tales como la warfarina). Su médico le pedirá que se someta a análisis de sangre regularmente y podrá ajustar la dosis para evitar un mayor riesgo de hemorragia.
- Vitaminas liposolubles (como las vitaminas A, D, E o K) o medicamentos altamente liposolubles (como los corticoides, ciclosporina, tacrolimus, antihistamínicos). Vedrop puede aumentar su absorción durante la digestión, por lo que el médico supervisará el efecto del tratamiento y ajustará la dosis cuando sea necesario.
Embarazo y lactancia
No existen datos clínicos disponibles de la utilización de este medicamento durante el embarazo. Informe a su médico si está embarazada para que decida si es conveniente utilizar el medicamento.
No existen datos acerca de si este medicamento se excreta en la leche materna. Informe a su médico si usted desea amamantar a su hijo para que decida si es conveniente utilizar el medicamento. Su médico le ayudará a tomar la mejor decisión para usted y su hijo.
Consulte a su médico o farmacéutico antes de tomar cualquier medicamento.
Conducción y uso de máquinas
Es poco probable que Vedrop afecte a su capacidad para conducir y utilizar máquinas.
Vedrop contiene metil-parahidroxibenzoato sódico (E219) y etil- parahidroxibenzoato sódico (E215), que pueden producir reacciones alérgicas (posiblemente retardadas).
Vedrop contiene 0,18 mmoles (4,1 mg) de sodio por ml. Consulte a su médico si sigue una dieta pobre en sodio.
3. Cómo tomar Vedrop
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis habitual es de 0,34 ml/kg/día.
Su médico le recetará la dosis en ml.
El médico le modificará la dosis de este medicamento de acuerdo con sus niveles de vitamina E en sangre.
Forma de administración
Trague la solución con o sin agua. Úsela únicamente con la jeringa para uso oral que se incluye en el envase.
Puede tomar Vedrop antes o durante las comidas, con o sin agua.
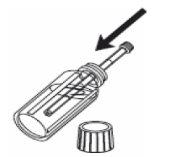
Para medir la dosis:
1- Abra el frasco. 2- Introduzca la jeringa de uso oral en el frasco. |
|
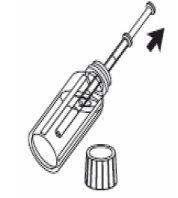
3- Llene la jeringa de uso oral con el líquido tirando del émbolo hasta la marca de referencia correspondiente a la cantidad en mililitros (ml) que le ha prescrito el médico. |
|
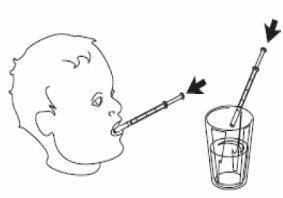
4- Retire la jeringa de uso oral del frasco. 5- Vacíe el contenido de la jeringa presionando sobre el émbolo hasta el fondo ya sea:
o
|
|
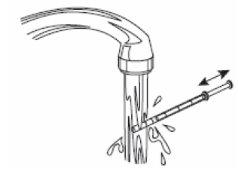
6- Cierre el frasco. 7- Lave la jeringa con agua. |
|
Si toma más Vedrop del que debe
Si toma dosis altas de vitamina E, puede padecer diarrea y dolor de estómago transitorios. Consulte a su médico o farmacéutico si los síntomas persisten más de dos días.
Si olvidó tomar Vedrop
No tome la dosis que ha olvidado y vuelva a la pauta de administración regular programada. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Vedrop
No interrumpa el tratamiento sin consultar a su médico porque puede volver a producirse una carencia de vitamina E que afecte a su salud. Póngase en contacto con su médico o farmacéutico antes de interrumpirlo.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se comunicaron los siguientes efectos adversos:
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Diarrea
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Astenia (sensación de debilidad)
- Cefalea
- Pérdida del cabello
- Picores
- Erupción cutánea
- Niveles anormales de sodio en sangre
- Niveles anormales de potasio en sangre
- Aumento de las transaminasas (enzimas hepáticas)
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- Dolor de estómago
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Anexo V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Vedrop
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el frasco después de EXP. La fecha de caducidad es el último día del mes que se indica.
- Este medicamento no requiere condiciones especiales de conservación.
- Desechar el medicamento un mes después que se haya abierto, aunque quede algo de solución.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Vedrop
- El principio activo es tocofersolán. Cada ml de solución contiene 50 mg de d-alfa-tocoferol en forma de tocofersolán, que equivalen a 74,5 UI de tocoferol.
- Los demás componentes son: sorbato potásico, metil-parahidroxibenzoato sódico (E219) y etil-parahidroxibenzoato sódico (E215) (ver el final de la sección 2 para obtener más información sobre estos 2 excipientes), glicerol, fosfato disódico dodecahidrato, ácido clorhídrico concentrado, agua ultrapurificada.
Aspecto de Vedrop y contenido del envase
Vedrop es una solución oral de color amarillo pálido ligeramente viscosa contenida en un frasco de cristal de color marrón, sellado con tapón de seguridad. Los frascos contienen 10 ml, 20 ml ó 60 ml de solución oral. Cada envase contiene un frasco y una jeringa de uso oral (una jeringa de 1 ml con un frasco de 10 ml ó 20 ml, una jeringa de 2 ml con un frasco de 60 ml).
Titular de la autorización de comercialización
Recordati Rare Diseases
Immeuble “Le Wilson”
70, avenue du General de Gaulle
F-92800 Puteaux
Francia
Responsable de la fabricación
Recordati Rare Diseases
Immeuble “Le Wilson”
70, avenue du Général de Gaulle
92800 Puteaux
Francia
o
Recordati Rare Diseases
Eco River Parc
30, rue des Peupliers
F-92000 Nanterre
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
Belgique/België/Belgien Recordati Tél/Tel: +32 2 46101 36 | Lietuva Recordati AB. Tel: + 46 8 545 80 230 Švedija |
???????? Recordati Rare Diseases Te?.: +33 (0)1 47 73 64 58 ??????? | Luxembourg/Luxemburg Recordati Tél/Tel: +32 2 46101 36 Belgique/Belgien |
Ceská republika Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francie | Magyarország Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Franciaország |
Danmark Recordati AB. Tlf : +46 8 545 80 230 Sverige | Malta Recordati Rare Diseases Tel: +33 1 47 73 64 58 Franza |
Deutschland Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 | Nederland Recordati Tel: +32 2 46101 36 België |
Eesti Recordati AB. Tel: + 46 8 545 80 230 Rootsi | Norge Recordati AB. Tlf : +46 8 545 80 230 Sverige |
Ελλ?δα Recordati Rare Diseases Τηλ: +33 1 47 73 64 58 Γαλλ?α | Österreich Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 Deutschland |
España Recordati Rare Diseases Spain S.L.U. Tel: + 34 91 659 28 90 | Polska Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francja |
France Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 | Portugal Jaba Recordati S.A. Tel: +351 21 432 95 00 |
Hrvatska Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 Francuska | România Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Franta |
Ireland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Slovenija Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francija |
Ísland Recordati AB. Simi:+46 8 545 80 230 Svíþjóð | Slovenská republika Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francúzsko |
Italia Recordati Rare Diseases Italy Srl Tel: +39 02 487 87 173 | Suomi/Finland Recordati AB. Puh/Tel : +46 8 545 80 230 Sverige |
Κ?προς Recordati Rare Diseases Τηλ : +33 1 47 73 64 58 Γαλλ?α | Sverige Recordati AB. Tel : +46 8 545 80 230 |
Latvija Recordati AB. Tel: + 46 8 545 80 230 Zviedrija | United Kingdom Recordati Rare Diseases UK Ltd. Tel: +44 (0)1491 414333 |
Fecha de la última revisión de este prospecto:
Este medicamento se ha autorizado en “circunstancias excepcionales”. Esta modalidad de aprobación significa que, debido a la rareza de su enfermedad, no ha sido posible obtener información completa de este medicamento.
La Agencia Europea de Medicamentos revisará anualmente la información nueva de este medicamento que pueda estar disponible y este prospecto se actualizará cuando sea necesario.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VEDROP 50 MG/ML EN SOLUCION ORALForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 50 mg/mlPrincipio activo: TocofersolanFabricante: Recordati Rare DiseasesRequiere recetaForma farmacéutica: CAPSULA, 2000 mgPrincipio activo: Tocopherol (vit E)Fabricante: Chiesi España S.A.U.Requiere recetaForma farmacéutica: CAPSULA, 400 mgPrincipio activo: Tocopherol (vit E)Fabricante: Chiesi España S.A.U.Requiere receta
Médicos online para VEDROP 50 MG/ML EN SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VEDROP 50 MG/ML EN SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes