
UPSTAZA 2,8 × 10E11 GENOMAS VECTORIALES (VG)/0,5 ML PARA SOLUCION PARA PERFUSION

Cómo usar UPSTAZA 2,8 × 10E11 GENOMAS VECTORIALES (VG)/0,5 ML PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Upstaza 2,8 × 1011genomas vectoriales/0,5 ml solución para perfusión
eladocagén exuparvovec
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que usted o su hijo pudieran tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de que a usted o a su hijo se le administre este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte al médico o enfermero.
- Si usted o su hijo experimenta efectos adversos, consulte al médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Upstaza y para qué se utiliza
- Qué necesita saber antes de que a usted o a su hijo se le administre Upstaza
- Cómo se administra Upstaza a usted o a su hijo
- Posibles efectos adversos
- Conservación de Upstaza
- Contenido del envase e información adicional
1. Qué es Upstaza y para qué se utiliza
Qué es Upstaza
Upstaza es un medicamento de terapia génica que contiene el principio activo eladocagén exuparvovec.
Para qué se utiliza Upstaza
Upstaza se utiliza para el tratamiento de pacientes de 18 meses y mayores, con una deficiencia de la proteína llamada L-aminoácido aromático descarboxilasa (AADC). Esta proteína es esencial para fabricar ciertas sustancias que el sistema nervioso del cuerpo necesita para funcionar correctamente.
La deficiencia de AADC es una afección hereditaria causada por una mutación (cambio) en el gen que controla la producción de AADC (también llamado gen de la dopa descarboxilasa o DDC). Esta afección impide el desarrollo del sistema nervioso del niño, lo que significa que muchas de las funciones del cuerpo no se desarrollan correctamente durante la infancia, como el movimiento, la alimentación, la respiración, el habla y la capacidad mental.
Cómo funciona Upstaza
El principio activo de Upstaza, eladocagén exuparvovec, es un tipo de virus llamado virus adenoasociado que ha sido modificado para incluir una copia del gen DDC que funciona correctamente. Upstaza se administra mediante perfusión (goteo) en una zona del cerebro llamada putamen, donde se fabrica el AADC. El virus adenoasociado permite que el gen DDC pase a las células del cerebro. De esta manera, Upstaza permite a las células producir AADC para que el organismo pueda fabricar las sustancias que necesita el sistema nervioso.
El virus adenoasociado utilizado para administrar el gen no provoca enfermedades en los seres humanos.
2. Qué necesita saber antes de que a usted o a su hijo se le administre Upstaza
Usted o su hijo no recibirán el tratamiento con Upstaza:
- si usted o su hijo son alérgicos a eladocagén exuparvovec a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
- Los movimientos espasmódicos incontrolables leves o moderados (también llamados discinesia) o los trastornos del sueño (insomnio) pueden aparecer o empeorar 1 mes después del tratamiento con Upstaza y perdurar durante varios meses más. El médico decidirá si usted o su hijo necesitan tratamiento para estos efectos.
- El médico monitorizará a usted o a su hijo para detectar complicaciones del tratamiento con Upstaza, como derrames del líquido que rodea el cerebro, meningitis o encefalitis.
- Durante los días siguientes a la intervención, el médico vigilará a su hijo para detectar posibles complicaciones como consecuencia de la intervención y de la anestesia general. Algunos de los síntomas de la enfermedad pueden verse amplificados durante ese periodo.
- Algunos síntomas específicos de la deficiencia de AADC pueden persistir después del tratamiento, algunos ejemplos de estos síntomas pueden ser las repercusiones en el estado de ánimo, la sudoración y la temperatura corporal.
- Después del tratamiento, algo del medicamento puede pasar a sus líquidos corporales o a los de su hijo (p. ej., lágrimas, sangre, secreciones nasales y líquido cefalorraquídeo); esto se conoce como “diseminación”. Usted o su hijo y la persona que lo cuida (especialmente si está embarazada, amamantando o inmunodeprimida) deben usar guantes y colocar los apósitos usados y otros materiales de desecho con lágrimas y secreciones nasales en bolsas selladas antes de tirarlos. Debe seguir estas precauciones durante 14 días.
- Usted o su hijo no deben donar sangre, órganos, tejidos ni células para trasplantes después del tratamiento con Upstaza, ya que Upstaza es un medicamento de terapia génica.
Niños y adolescentes
Upstaza no se ha estudiado en niños menores de 18 meses de edad. La experiencia es escasa en niños mayores de 12 años.
Otros medicamentos y Upstaza
Informe a su médico si usted o su hijo están tomando, han tomado recientemente o pudieran tener que tomar cualquier otro medicamento.
Usted o su hijo pueden recibir las vacunas infantiles habituales con normalidad.
Embarazo, lactancia y fertilidad
Se desconocen los efectos de este medicamento en el embarazo y el feto.
Upstaza no se ha estudiado en mujeres en periodo de lactancia.
No hay información sobre el efecto de Upstaza en la fertilidad masculina o femenina.
Upstaza contiene sodio y potasio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
Este medicamento contiene menos de 1 mmol de potasio (39 mg) por dosis; esto es, esencialmente “exento de potasio”.
3. Cómo se administra Upstaza a usted o a su hijo
- Usted o su hijo recibirán Upstaza en el quirófano por parte de neurocirujanos con experiencia en cirugía cerebral.
- Upstaza se administra bajo anestesia. El neurocirujano hablará con usted acerca de la anestesia y de cómo se administra.
- Antes de administrar Upstaza, el neurocirujano hará dos orificios pequeños en su cráneo o en el de su hijo, uno a cada lado.
- A continuación, se inyectará Upstaza a través de estos orificios en cuatro puntos de su cerebro o en el de su hijo, en una zona denominada putamen.
- Tras la perfusión, se cerrarán los dos orificios y usted o su hijo se someterán a un escáner cerebral.
- Usted o su hijo tendrán que permanecer en el hospital o cerca de este durante unos días para supervisar la recuperación y comprobar si hay efectos secundarios de la intervención quirúrgica o la anestesia
- El médico le verá a usted o a su hijo en el hospital dos veces, una aproximadamente a la semana después de la intervención quirúrgica, y otras tres semanas después de esta, para hacer un seguimiento de la recuperación y comprobar si hay algún efecto secundario de la intervención quirúrgica y el tratamiento.
Si a usted o a su hijo se les administra más Upstaza del que se debe
Como este medicamento se lo administra a usted o a su hijo un médico, es poco probable que usted o su hijo reciban una cantidad excesiva. Si esto ocurre, el médico tratará los síntomas, según sea necesario.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Pueden aparecer los siguientes efectos adversos relacionados con Upstaza:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Discinesia (movimientos espasmódicos incontrolables)
- Insomnio (dificultad para dormir), irritabilidad
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Aumento de la producción de saliva
Pueden aparecer los siguientes efectos adversos relacionados con la intervención quirúrgica para administrar Upstaza:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Nivel bajo de glóbulos rojos (anemia)
- Derrame del líquido que rodea el cerebro (llamado líquido cefalorraquídeo) (los posibles síntomas son dolor de cabeza, náuseas y vómitos, dolor o rigidez de cuello, cambios en la audición, sensación de desequilibrio, mareos o vértigo)
Los siguientes efectos secundarios pueden producirse en las dos semanas siguientes a la intervención quirúrgica a la administración de Upstaza, debido a la anestesia o a los efectos posoperatorios:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Hemorragia gastrointestinal, diarrea
- Fiebre, ruidos respiratorios anómalos
- Neumonía
- Nivel bajo de potasio en sangre
- Irritabilidad
- Hipotensión (presión arterial baja)
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Cianosis (coloración azulada de la piel causada por la falta de oxígeno en la sangre)
- Ulceración de la boca
- Hipotermia (temperatura corporal baja)
- Gastroenteritis
- Discinesia (movimientos espasmódicos incontrolables)
- Insuficiencia respiratoria
- Úlcera por presión, dermatitis del pañal, erupción cutánea
- Extracción de dientes
- Shock hipovolémico (pérdida grave de sangre o líquidos corporales)
Comunicación de efectos adversos
Si usted o su hijo experimentan cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Upstaza
La siguiente información está destinada únicamente a los médicos.
Upstaza se conservará en el hospital. Debe conservarse y transportarse congelado a temperatura ≤ -65 ºC. Se descongela antes de usarlo y, una vez descongelado, tiene que usarse antes de 6 horas. No debe volverse a congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD.
6. Contenido del envase e información adicional
Composición de Upstaza
- El principio activo es eladocagén exuparvovec. Cada 0,5 ml de solución contiene 2,8 × 1011 genomas vectoriales de eladocagén exuparvovec.
Los demás componentes son cloruro de potasio, cloruro de sodio, dihidrógeno fosfato de potasio, hidrógenofosfato de disodio, poloxámero 188 y agua para preparaciones inyectables (ver sección 2 “Upstaza contiene sodio y potasio”).
Aspecto del producto y contenido del envase
Upstaza es una solución para perfusión, clara o ligeramente opalescente, incolora o blanca tenue, que se presenta en un vial de vidrio transparente.
Cada envase contiene 1 vial.
Titular de la autorización de comercialización
PTC Therapeutics International Limited
70 Sir John Rogerson's Quay
Dublín 2
Irlanda
Responsable de la fabricación
Almac Pharma Services (Irlanda) Limited
Finnabair Industrial Estate
Dundalk, Co. Louth, A91 P9KD
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
AT, BE, BG, CY, CZ, DK, DE, EE, EL, ES, HR, HU, IE, IS, IT, LT, LU, LV, MT, NL, NO, PL, PT, RO, SI, SK, FI, SE, UK (NI) PTC Therapeutics International Ltd. (Irlanda) +353 (0)1 447 5165 | FR PTC Therapeutics France Tel: +33(0)1 76 70 10 01 |
Fecha de la última revisión de este prospecto.
Este medicamento se ha autorizado en “circunstancias excepcionales”. Esta modalidad de aprobación significa que debido a la rareza de esta enfermedad no ha sido posible obtener información completa de este medicamento.
La Agencia Europea de Medicamentos revisará anualmente la información nueva de este medicamento que pueda estar disponible y este prospecto se actualizará cuando sea necesario.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Esta información está destinada únicamente a profesionales sanitarios:
Instrucciones sobre preparación, administración, medidas tomadas en caso de exposición accidental y eliminación de Upstaza
Cada vial es para un solo uso. Este medicamento solo debe inyectarse con la cánula ventricular SmartFlow.
Precauciones que se deben tomar antes de manipular o administrar el medicamento
Este medicamento contiene virus modificados genéticamente. Durante la preparación, administración y eliminación, se debe usar un equipo de protección personal (que incluya bata, gafas de seguridad, mascarilla y guantes) cuando se manipulen eladocagén exuparvovec y los materiales que hayan estado en contacto con la solución (residuos sólidos y líquidos).
Descongelación en la farmacia del hospital
- Upstaza se entrega a la farmacia congelado y debe conservarse en el embalaje exterior a temperatura ≤ -65 ºC hasta que se prepare para su uso.
- Upstaza debe manipularse de forma aséptica y bajo condiciones estériles.
- Deje que el vial congelado de Upstaza se descongele en posición vertical a temperatura ambiente hasta que el contenido esté completamente descongelado. Invierta suavemente el vial unas 3 veces, NO agitar.
- Examine Upstaza después de mezclarlo. Si se observan partículas, turbidez o cambio de color, no utilice el producto.
Preparación antes de la administración
- Transfiera el vial, la jeringa, la aguja, el capuchón de la jeringa, las bolsas estériles o los envoltorios estériles respetando el procedimiento del hospital para la transferencia y el uso de la jeringa llena en el quirófano previsto, y etiquetándolo en la cabina de seguridad biológica (BSC). Use guantes estériles y otros equipos de protección personal (que incluya bata, gafas de seguridad y mascarilla) según el procedimiento normal de trabajo para BSC.
- Abra la jeringa de 5 ml (jeringa de polipropileno de 5 ml con émbolo de elastómero sin látex, lubricado con aceite de silicona de grado médico) y etiquétela adecuadamente como jeringa llena de producto según el procedimiento de la farmacia y las normativas locales.
- Acople la aguja de calibre 18 o 19 con filtro (agujas con filtro de 5 µm de acero inoxidable de 1,5 pulgadas y calibre 18 o 19) a la jeringa.
- Introduzca todo el volumen del vial de Upstaza en la jeringa. Invierta el vial y la jeringa y retire parcialmente o incline la aguja según sea necesario para maximizar la recuperación del producto.
- Aspire aire en la jeringa para que la aguja se vacíe de producto. Retire con cuidado la aguja de la jeringa de 5 ml que contiene Upstaza. Purgue el aire de la jeringa hasta que no haya ninguna burbuja de aire y luego tape con un capuchón de jeringa.
- Envuelva la jeringa en una bolsa de plástico estéril (o en varias bolsas según el procedimiento hospitalario habitual) y colóquela en un recipiente secundario adecuado (por ejemplo, una nevera de plástico duro) para llevarla al quirófano a temperatura ambiente. El uso de la jeringa (es decir, la conexión de la jeringa al dispensador de la jeringa y el inicio del cebado de la cánula) debe comenzar en un plazo de 6 horas desde el inicio de la descongelación del producto.
Administración en el quirófano
- Acople firmemente la jeringa que contiene Upstaza a la cánula ventricular SmartFlow.
- Inserte la jeringa de Upstaza en una bomba de perfusión compatible con la jeringa de 5 ml. Bombee Upstaza a 0,003 ml/min hasta que la primera gota de Upstaza pueda verse en la punta de la aguja. Deténgase y espere hasta que esté listo para la perfusión.
Precauciones que deben tomarse para la eliminación del medicamento y la exposición accidental
- Debe evitarse la exposición accidental a eladocagén exuparvovec, incluido el contacto con la piel, los ojos y las membranas mucosas.
- En caso de exposición a la piel, la zona afectada debe limpiarse a fondo con agua y jabón durante al menos 5 minutos. En caso de contacto con los ojos, la zona afectada debe enjuagarse bien con agua durante al menos 5 minutos.
- En caso de lesión por pinchazo, la zona afectada debe limpiarse bien con agua y jabón o un desinfectante.
- Todo el eladocagén exuparvovec que no se haya usado o los materiales residuales deben eliminarse de acuerdo con las normativas locales sobre residuos farmacéuticos.
- Los posibles vertidos deben limpiarse con una gasa absorbente y desinfectarse con una solución de lejía, seguida del uso de toallitas con alcohol.
- Tras la administración, el riesgo de diseminación se considera bajo. Se recomienda asesorar a los cuidadores y a los familiares de los pacientes para que sigan las precauciones adecuadas para la manipulación de los líquidos corporales y los residuos del paciente durante los 14 días posteriores a la administración de eladocagén exuparvovec (ver sección 4.4 de la ficha técnica/resumen de las características del producto).
Posología
El tratamiento debe ser administrado en un centro especializado en neurocirugía estereotáctica, por un neurocirujano cualificado bajo condiciones asépticas controladas.
Los pacientes recibirán una dosis total de 1,8 × 1011 vg administrada en cuatro perfusiones de 0,08 ml (0,45 × 1011 vg) (dos por putamen).
La posología es la misma para toda la población contemplada en la indicación.
Forma de administración
Vía intraputaminal.
La administración de Upstaza puede provocar un derrame de líquido cefalorraquídeo después de la intervención quirúrgica. Se debe hacer un seguimiento minucioso de los pacientes que reciben tratamiento con Upstaza después de su administración.
Administración neuroquirúrgica
Upstaza es un vial de un solo uso administrado mediante inyección intraputaminal bilateral en una sesión quirúrgica en dos puntos del putamen. Se aplican cuatro inyecciones distintas de volúmenes iguales en el putamen anterior derecho, el putamen posterior derecho, el putamen anterior izquierdo y el putamen posterior izquierdo.
Siga los pasos siguientes para administrar Upstaza:
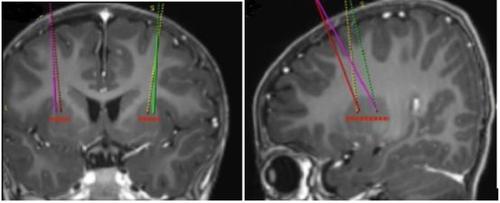
- Los puntos de perfusión diana se definen según la práctica neuroquirúrgica estereotáctica de referencia. Upstaza se administra como perfusión bilateral (2 perfusiones por putamen) con una cánula intracraneal. Los 4 puntos finales de cada trayectoria deben definirse como 2 mm en dirección posterior (por encima) de los puntos diana anteriores y posteriores en el plano medio-horizontal (Figura 1).
Figura 1 Cuatro lugares deseados para los puntos de inyección
- Una vez completado el registro estereotáctico, debe marcarse el punto de entrada en el cráneo. Debe realizarse el acceso quirúrgico a través del hueso del cráneo y la duramadre.
- La cánula de perfusión se coloca en el punto designado del putamen con medios estereotácticos a partir de las trayectorias previstas. Cabe destacar que se coloca la cánula de perfusión y se realiza la perfusión por separado para cada putamen.
- Upstaza se inyecta a una velocidad de 0,003 ml/min en cada uno de los 2 puntos diana de cada putamen; se inyectan 0,08 ml de Upstaza por punto del putamen, lo que da lugar a 4 perfusiones con un volumen total de 0,320 ml (o 1,8 × 1011 vg).
- Empezando por el primer punto diana, la cánula se inserta a través de un orificio de trepanación en el putamen y luego se retira lentamente, distribuyendo los 0,08 ml de Upstaza a través de la trayectoria prevista para optimizar la distribución en el putamen.
- Tras la primera perfusión, se retira la cánula y se vuelve a introducir en el siguiente punto diana, repitiendo el mismo procedimiento para los otros 3 puntos diana (anterior y posterior de cada putamen).
- Tras los procedimientos de cierre neuroquirúrgico habituales, el paciente se somete a un examen de imagen por tomografía computarizada posoperatoria para comprobar que no haya complicaciones posoperatorias (por ej., hemorragias).
- El paciente debe alojarse en las inmediaciones del hospital en el que se ha realizado la intervención, como mínimo durante las primeras 48 horas después de esta. El paciente puede volver a casa, después de la intervención, según el criterio del médico responsable. La atención posterior al tratamiento debe ser dirigida por el neurólogo pediátrico de referencia y por el neurocirujano. Se realizará un seguimiento al paciente a los 7 días después de la intervención para comprobar que no hayan surgido complicaciones. Dos semanas más tarde, tendrá lugar una segunda visita de seguimiento, es decir, a las 3 semanas después de la intervención para supervisar la recuperación posquirúrgica y la presencia de acontecimientos adversos.
- Se ofrecerá a los pacientes la posibilidad de inscribirse en un registro para seguir evaluando la seguridad y la eficacia a largo plazo del tratamiento en condiciones normales de la práctica clínica.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a UPSTAZA 2,8 × 10E11 GENOMAS VECTORIALES (VG)/0,5 ML PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 100 UPrincipio activo: LaronidasaFabricante: Sanofi B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 30 mg/mlPrincipio activo: Cerliponasa alfaFabricante: Biomarin International LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, DesconocidaPrincipio activo: ImiglucerasaFabricante: Sanofi B.V.Requiere receta
Médicos online para UPSTAZA 2,8 × 10E11 GENOMAS VECTORIALES (VG)/0,5 ML PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de UPSTAZA 2,8 × 10E11 GENOMAS VECTORIALES (VG)/0,5 ML PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















