SUNLENCA 464 MG SOLUCION INYECTABLE

Cómo usar SUNLENCA 464 MG SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Sunlenca 464 mg solución inyectable
lenacapavir
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Sunlenca y para qué se utiliza
- Qué necesita saber antes de que le administren Sunlenca
- Cómo se administra Sunlenca
- Posibles efectos adversos
- Conservación de Sunlenca
- Contenido del envase e información adicional
1. Qué es Sunlenca y para qué se utiliza
Sunlenca contiene el principio activo lenacapavir. Se trata de un medicamento antirretroviral conocido como un inhibidor de la cápside.
Sunlenca es un medicamento de acción prolongada y se utiliza en combinación con otros medicamentos antirretroviralespara tratar el virus de la inmunodeficiencia humana tipo 1 (VIH), el virus que causa el síndrome de inmunodeficiencia adquirida (sida).
Se utiliza para tratar la infección por el VIH en adultos con opciones de tratamiento limitadas (por ejemplo, cuando otros medicamentos antirretrovirales no son lo suficientemente eficaces o no son adecuados).
El tratamiento con Sunlenca en combinación con otros antirretrovirales reduce la cantidad de VIH presente en el organismo. Esto mejorará la función del sistema inmunitario (las defensas naturales del organismo) y disminuirá el riesgo de desarrollar enfermedades asociadas a la infección por el VIH.
2. Qué necesita saber antes de que le administren Sunlenca
No reciba Sunlenca
- Si es alérgico a lenacapavir o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si está tomando alguno de los siguientes medicamentos:
- rifampicina, utilizada para tratar algunas infecciones bacterianas como la tuberculosis
- carbamazepina, fenitoína, utilizadas para prevenir las crisis convulsivas
- hierba de San Juan (Hypericum perforatum), un medicamento a base de plantas utilizado para la depresión y la ansiedad
?Si cree que esto le aplica, no reciba Sunlenca e informe a su médico inmediatamente.
Advertencias y precauciones
Consulte a su médico antes de usar Sunlenca
- Consulte a su médico o farmacéutico si tiene o alguna vez ha tenido enfermedad hepática grave o si las pruebas han mostrado problemas de hígado. Su médico evaluará detenidamente si tratarle o no con Sunlenca.
Mientras esté usando Sunlenca
Una vez que empiece a usar Sunlenca, esté atento a:
- Signos de inflamación o infección.
?Si nota cualquiera de estos síntomas, informe a su médico inmediatamente.Para más información, ver sección 4, Posibles efectos adversos.
Las citas periódicas son importantes
Es importante que acuda a sus citas programadaspara recibir la inyección de Sunlenca para controlar la infección por el VIH y evitar que su enfermedad empeore. Hable con su médico si está considerando interrumpir el tratamiento. Si se produce un retraso en la administración de su inyección o si deja de recibir Sunlenca, tendrá que tomar otros medicamentos para tratar la infección por el VIH y reducir el riesgo de desarrollar resistencia viral.
Niños y adolescentes
No administre este medicamento a niños menores de 18 años de edad. No se ha estudiado todavía el uso de Sunlenca en pacientes menores de 18 años de edad, por lo que se desconoce cómo de seguro y eficaz es el medicamento en este grupo de edad.
Otros medicamentos y Sunlenca
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Sunlenca puede interaccionar con otros medicamentos. Esto puede impedir que Sunlenca u otros medicamentos funcionen correctamente o empeorar los efectos adversos. En algunos casos, su médico puede tener que ajustar la dosis o comprobar sus concentraciones sanguíneas.
Medicamentos que nunca se deben tomar con Sunlenca:
- rifampicina, utilizada para tratar algunas infecciones bacterianas como la tuberculosis
- carbamazepina, fenitoína, utilizadas para prevenir las crisis convulsivas
- hierba de San Juan (Hypericum perforatum), un medicamento a base de plantas utilizado para la depresión y la ansiedad
?.Si está tomando alguno de estos medicamentos, no reciba Sunlenca inyectable e informe a su médico inmediatamente.
Consulte a su médico especialmente si está tomando:
- antibióticos que contengan:
- rifabutina
- antiepilépticos, utilizados para tratar la epilepsia y prevenir las crisis convulsivas (convulsiones), que contengan:
- oxcarbazepina o fenobarbital
- medicamentos utilizados para tratar el VIH, que contengan:
- atazanavir/cobicistat, efavirenz, nevirapina, tipranavir/ritonavir o etravirina
- medicamentos utilizados para tratar la cefalea migrañosa, que contengan:
- dihidroergotamina o ergotamina
- medicamentos utilizados para tratar la impotencia y la hipertensión pulmonar, que contengan:
- sildenafilo o tadalafilo
- medicamentos utilizados para tratar la impotencia, que contengan:
- vardenafilo
- corticoesteroides (también conocidos como “esteroides”) administrados por vía oral o mediante inyección, utilizados para tratar alergias, enfermedades inflamatorias intestinales y otras enfermedades diversas que implican inflamación en el organismo, que contengan:
- dexametasona o hidrocortisona/cortisona
- medicamentos utilizados para reducir el colesterol, que contengan:
- lovastatina o simvastatina
- antiarrítmicos utilizados para tratar problemas cardíacos, que contengan:
- digoxina
- medicamentos utilizados para ayudarle a dormir, que contengan:
- midazolam o triazolam
- anticoagulantes utilizados para prevenir y tratar los coágulos de sangre, que contengan:
- rivaroxabán, dabigatrán o edoxabán
?Informe a su médico si está tomando alguno de estos medicamentoso si empieza a tomar alguno de estos medicamentos durante el tratamiento con Sunlenca. No interrumpa su tratamiento sin consultar con su médico.
Sunlenca es un medicamento de acción prolongada. Debe saber que, si decide interrumpir o cambiar su tratamiento a otro tras consultar a su médico, concentraciones bajas de lenacapavir (el principio activo de Sunlenca) pueden permanecer en su organismo durante varios meses después de su última inyección. No se prevé que la presencia de estas concentraciones bajas restantes afecte a otros medicamentos antirretrovirales que tome posteriormente para tratar la infección por el VIH. Sin embargo, la presencia de concentraciones bajas de lenacapavir en su organismo puede afectar a otros medicamentos que tome en los 9 meses siguientes a su última inyección de Sunlenca. Pregunte a su médico si esos medicamentos son seguros para usted tras interrumpir el tratamiento con Sunlenca.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Como medida de precaución, debe evitar el uso de Sunlenca durante el embarazo a menos que su médico le indique lo contrario.
Se recomienda que las mujeres que presentan infección por el VIH no den el pecho a sus hijos, puesto que la infección por el VIH-1 se puede transmitir al bebé a través de la leche materna. Si está amamantando o tiene intención de amamantar, hable con su médico lo antes posible.
Conducción y uso de máquinas
No se prevé que Sunlenca afecte a su capacidad para conducir o utilizar máquinas.
Sunlenca contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por inyección; esto es, esencialmente “exento de sodio”.
3. Cómo se administra Sunlenca
Sunlenca se utiliza en combinación con otros medicamentos antirretrovialespara tratar la infección por el VIH. Su médico le indicará qué otros medicamentos debe tomar para tratar la infección por el VIH y cuándo debe tomarlos.
El tratamiento con Sunlenca comienza con la toma de comprimidos por vía oral, seguido de inyecciones administradas por su médico o enfermero, tal y como se describe a continuación.
Consulte a su médico antes de tomar los comprimidos.El médico le informará cuándo debe empezar a tomar los comprimidos y para cuándo se programará su cita para recibir las primeras inyecciones.
Día 1 de tratamiento:
- Dos comprimidos por vía oral. Estos se pueden tomar con o sin alimentos.
Día 2 de tratamiento:
- Dos comprimidos por vía oral. Estos se pueden tomar con o sin alimentos.
Día 8 de tratamiento:
- Un comprimido por vía oral. Este se puede tomar con o sin alimentos.
Día 15 de tratamiento:
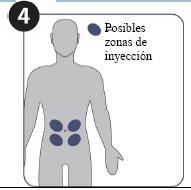
- Dos inyecciones en el abdomen (el vientre) administradas al mismo tiempo por su médico o enfermero.
Cada 6 meses:
- Dos inyecciones en el abdomen administradas al mismo tiempo por su médico o enfermero.
Si le administran más Sunlenca inyectable del que se debe
Su médico o un enfermero le administrará este medicamento, por lo tanto, es poco probable que le administren demasiado. Informe al médico o al enfermero si le preocupa.
Si olvidó una inyección de Sunlenca
- Es importante que acuda a sus citas programadas cada 6 meses para recibir las inyecciones de Sunlenca. Esto ayudará a controlar la infección por el VIH y a evitar que su enfermedad empeore.
- Si cree que no podrá acudir a su cita para recibir las inyecciones, llame a su médico lo antes posible para comentar sus opciones de tratamiento.
Consulte el prospecto de Sunlenca comprimidos si olvidó tomar o vomita los comprimidos.
Si interrumpe el tratamiento con Sunlenca
No interrumpa el tratamiento con Sunlenca sin hablar con su médico. Continúe el tratamiento con las inyecciones de Sunlenca mientras su médico lo recomiende. Interrumpir Sunlenca puede afectar gravemente al funcionamiento de tratamientos futuros para el VIH.
?Hable con su médico si desea interrumpir el tratamiento con las inyecciones de Sunlenca.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Posibles efectos adversos graves: informe a un médico inmediatamente
- Cualquier signo de inflamación o de infección. En algunos pacientes con infección avanzada por el VIH (sida) y antecedentes de infecciones oportunistas (infecciones que ocurren en personas con un sistema inmunitario débil), se pueden producir signos y síntomas de inflamación por infecciones previas poco después de iniciar el tratamiento contra el VIH. Se cree que estos síntomas se deben a una mejoría de la respuesta inmunitaria del organismo, que le permite combatir infecciones que podrían haber estado presentes sin síntomas obvios.
- Se pueden producir también trastornos autoinmunitarios, en los que el sistema inmunitario ataca a los tejidos sanos del organismo, después de que empiece a tomar medicamentos para la infección por el VIH. Los trastornos autoinmunitarios se pueden producir muchos meses después del inicio del tratamiento. Esté atento a cualquier síntoma de infección u otros síntomas, como:
- debilidad muscular
- debilidad del organismo que se inicia en las manos y los pies y se desplaza hacia el tronco
- palpitaciones, temblor o hiperactividad
?Si nota cualquiera de estos síntomas o cualquier síntoma de inflamación o infección, informe a su médico inmediatamente.
Efectos adversos muy frecuentes
(pueden afectar a más de 1 de cada 10 personas)
- Reacciones donde se inyecta Sunlenca.
Los síntomas pueden incluir:
- dolor y molestias
- un bulto o masa endurecida
- reacción inflamatoria como enrojecimiento, picor e hinchazón
Efectos adversos frecuentes
(pueden afectar hasta 1 de cada 10 personas)
- Náuseas
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Sunlenca
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del vial y en la caja después de {CAD}. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna temperatura especial de conservación. Conservar en el embalaje original para protegerlo de la luz.
6. Contenido del envase e información adicional
Composición de Sunlenca
El principio activo es lenacapavir. Cada vial monodosis contiene 463,5 mg de lenacapavir.
Los demás componentes son
Macrogol (E1521), agua para preparaciones inyectables.
Aspecto de Sunlenca y contenido del envase
Sunlenca solución inyectable (inyectable) es una solución transparente, de amarilla a marrón, sin partículas visibles. Sunlenca se presenta en dos viales de vidrio que contienen 1,5 ml de solución inyectable cada uno. Estos viales vienen incluidos en un kit de administración que también contiene 2 dispositivos de acceso al vial (un dispositivo que permitirá a su médico o enfermero extraer Sunlenca del vial), 2 jeringas desechables y 2 agujas para inyección.
Titular de la autorización de comercialización
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Irlanda
Responsable de la fabricación
Gilead Sciences Ireland UC
IDA Business & Technology Park
Carrigtohill
County Cork
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Gilead Sciences Belgium SRL-BV Tél/Tel: + 32 (0) 24 01 35 50 | Lietuva Gilead Sciences Poland Sp. z o.o. Tel.: + 48 22 262 8702 |
???????? Gilead Sciences Ireland UC ???.: + 353 (0) 1 686 1888 | Luxembourg/Luxemburg Gilead Sciences Belgium SRL-BV Tél/Tel: + 32 (0) 24 01 35 50 |
Ceská republika Gilead Sciences s.r.o. Tel: + 420 910 871 986 | Magyarország Gilead Sciences Ireland UC Tel.: + 353 (0) 1 686 1888 |
Danmark Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Deutschland Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Nederland Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Eesti Gilead Sciences Poland Sp. z o.o. Tel.: +48 22 262 8702 | Norge Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Ελλ?δα Gilead Sciences Ελλ?ς Μ.ΕΠΕ. Τηλ: + 30 210 8930 100 | Österreich Gilead Sciences GesmbH Tel: + 43 1 260 830 |
España Gilead Sciences, S.L. Tel: + 34 91 378 98 30 | Polska Gilead Sciences Poland Sp. z o.o. Tel.: + 48 22 262 8702 |
France Gilead Sciences Tél: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 21 7928790 |
Hrvatska Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | România Gilead Sciences (GSR) S.R.L. Tel: + 40 31 631 18 00 |
Ireland Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Slovenija Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Ísland Gilead Sciences Sweden AB Sími: + 46 (0) 8 5057 1849 | Slovenská republika Gilead Sciences Slovakia s.r.o. Tel: + 421 232 121 210 |
Italia Gilead Sciences S.r.l. Tel: + 39 02 439201 | Suomi/Finland Gilead Sciences Sweden AB Puh/Tel: + 46 (0) 8 5057 1849 |
Κ?προς Gilead Sciences Ελλ?ς Μ.ΕΠΕ. Τηλ: + 30 210 8930 100 | Sverige Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Latvija Gilead Sciences Poland Sp. z o.o. Tel.: + 48 22 262 8702 | United Kingdom (Northern Ireland) Gilead Sciences Ireland UC Tel: + 44 (0) 8000 113 700 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
-----------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
Instrucciones de uso-Sunlenca 464 mg solución inyectable
El kit contiene
2 viales |
|
2 dispositivos de acceso al vial |
|
2 jeringas |
|
2 agujas para inyección |
|
Todos los componentes son para un solo uso.
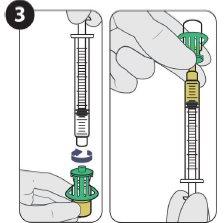
Se necesitan dos inyecciones de 1,5 mlpara una dosis completa. Es necesario utilizar el dispositivo de acceso al vial.
Compruebe que:
- El vial contiene una solución de amarilla a marrón, sin partículas
- El contenido no está dañado
- El medicamento no ha caducado
| |
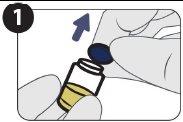
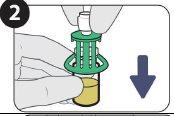
| Retire el tapón. |
| Limpie el precinto del vial con una toallita empapada en alcohol. |
| |
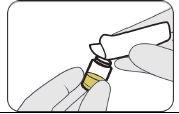
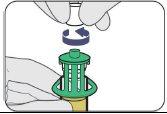
| Empuje hacia abajo. |
| Gírelo para retirarlo. |
| |
|
|
| |
| Posibles zonas de inyección (a un mínimo de 5 cm del ombligo) |
| |
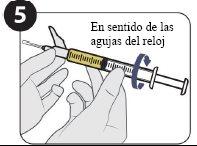
| Acople la aguja para inyección y llene 1,5 ml. |
| |
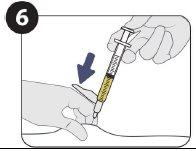
| Inyecte 1,5 ml de Sunlenca por vía subcutánea |
| |
| Repita los pasos para la 2.a inyección en una nueva zona de inyección. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SUNLENCA 464 MG SOLUCION INYECTABLEForma farmacéutica: COMPRIMIDO, 300 mgPrincipio activo: lenacapavirFabricante: Gilead Sciences Ireland Unlimited CompanyRequiere recetaForma farmacéutica: COMPRIMIDO, 150 mgPrincipio activo: MaravirocFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 300 mgPrincipio activo: MaravirocFabricante: Viiv Healthcare B.V.Requiere receta
Médicos online para SUNLENCA 464 MG SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SUNLENCA 464 MG SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes