LIDBREE 42 MG/ML GEL INTRAUTERINO


Cómo usar LIDBREE 42 MG/ML GEL INTRAUTERINO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO
Prospecto:información para el paciente
Lidbree 42 mg/ml gel intrauterino
lidocaína
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Lidbree y para qué se utiliza
- Qué necesita saber antes de empezar a usar Lidbree
- Cómo usar Lidbree
- Posibles efectos adversos
5 Conservación de Lidbree
- Contenido del envase e información adicional
1. Qué es Lidbree y para qué se utiliza
Lidbree es un gel anestésico utilizado para prevenir el dolor de los procedimientos ginecológicos, como la colocación de dispositivos anticonceptivos en el útero y el muestreo de biopsias para la evaluación de laboratorio en los exámenes ginecológicos, en adultos y adolescentes a partir de los 15 años de edad. Contiene el principio activo lidocaína, un anestésico local tipo amida (que adormece las partes del cuerpo a las que se aplica).
¿Cómo actúa Lidbree?
Después de la aplicación de gel, se requieren de 2 a 5 minutos antes de que el área genital (la mucosa) esté adormecida. Se ha demostrado que el gel reduce el dolor durante los procedimientos ginecológicos y hasta al menos 30 minutos después del procedimiento. Después de 1 hora, el efecto analgésico desaparece.
2. Qué necesita saber antes de empezar a usar Lidbree
No use Lidbree
Si es alérgico a la lidocaína o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Uso exclusivo por vía endocervical e intrauterina. Después del uso del gel para la colocación de un anticonceptivo intrauterino (dispositivo anticonceptivo intrauterino, DIU), en casos de inserción difícil, puede producirse sangrado y/o dolor excepcional. En esos casos, se debe realizar inmediatamente una exploración física y una ecografía para descartar una perforación del útero (matriz) o del cuello uterino (cuello de la matriz). El promedio es de 1 perforación por cada 1.000 procedimientos de inserción de DIU.
Informe al profesional que le administrará Lidbree:
- si tiene una alteración del ritmo cardíaco (bloqueo parcial o completo de la conducción cardíaca) ya que los anestésicos locales pueden afectarlo;
- si está recibiendo tratamiento para una alteración del ritmo cardíaco (con los llamados bloqueadores de los canales de potasio o antiarrítmicos de clase III (por ejemplo, amiodarona)) porque los efectos cardíacos pueden aumentar;
- si tiene una afección llamada porfiria aguda (una afección que se encuentra en la familia, relación con una de las proteínas en la sangre). La lidocaína puede causar ataques de porfiria y solo debe prescribirse en pacientes que padecen porfiria aguda en casos urgentes o necesarios;
- si tiene un mal estado de salud general.
Niños y adolescentes
Este medicamento no debe administrarse en niños menores de 15 años debido al riesgo de efectos secundarios producido por las altas concentraciones de lidocaína en sangre.
Otros medicamentos y Lidbree
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento que contenga lidocaína, o medicamentos para tratar el ritmo cardiaco irregular (antiarritmicos, como mexiletina o antiarritmicos clase III como la amiodarona), ya que sus efectos sobre el corazón se adicionarían a los efectos de la lidocaína.
Embarazo, lactancia y fertilidad
En base a la experiencia a largo plazo, se desconoce que la lidocaína produzca efectos adversos en el recién nacido.
La lidocaína se puede secretar en la leche materna, pero en cantidades tan pequeñas que generalmente no hay riesgo de que esto afecte al recién nacido. Por lo tanto, la lactancia materna puede continuar en caso de tratamiento con Lidbree
Se desconoce el efecto de la lidocaína sobre la fertilidad.
Conducción y uso de máquinas
Lidbree tiene una influencia nula o insignificante sobre la capacidad para conducir y utilizar máquinas.
Lidbree contiene ricinoleato de macrogolglicerol (aceite de ricino polioxil), y butilato de hidroxitolueno
El ricinoleato de macrogolglicerol puede producir reacciones alérgicas graves.
Este medicamento puede producir reacciones locales en la piel (como dermatitis de contacto) o irritación de los ojos y membranas mucosas porque contiene butilhidroxitolueno (E321).
3. Cómo usar Lidbree
Su médico o enfermero aplicará el gel anestésico paso a paso comenzando por la entrada del útero.
Uso enadolescentes
Los adolescentes de bajo peso, por debajo de 30 kg de peso corporal, deben recibir una dosis reducida.
Si usa más Lidbree del que debe
Con las dosis recomendadas no se espera que se produzcan los siguientes efectos, sin embargo, informe a su médico o enfermero inmediatamente si experimenta adormecimiento de los labios o la lengua, mareo, zumbido en el oído (tinnitus) o tiene dificultad para hablar o ver correctamente (trastornos visuales), ya que podrían ser los primeros síntomas de altas concentraciones de lidocaína en sangre. En ocasiones, pueden producirse espasmos o temblores musculares (temblores) o interrupciones de la respiración (apnea), en ese caso su médico o enfermero deben asegurarse con rapidez de que respira correctamente (soporte de las vías respiratorias) y administrarle anticonvulsivos.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, telefóno 91 562 04 20, indicando el medicamento y la cantidad utilizada o ingerida.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos secundarios experimentados después del uso de Lidbree en la inserción de anticonceptivos en el útero (matriz) son similares a los que se producen en el mismo proceso sin la aplicación de Lidbree.
Los posibles efectos adversos son:
- Muy frecuentes(más de 1 de cada 10 personas): náuseas (sensación de malestar).
- Frecuentes(pueden afectar hasta 1 de cada 10 personas): mareo, dolor de cabeza, sensaciones desagradables en el abdomen.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Lidbree
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad (mes-año) que aparece en la caja y en la jeringa. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Lidbree
- El principio activo es lidocaína. Cada ml de gel intrauterino contiene 42 mg de lidocaína.
- Los demás componentes son:
- Ricinoleato de macrogolglicerol (aceite de ricino polioxil)
- Poloxámero (contiene butilhidroxitolueno (E 321))
- Ascorbato de sodio (E 301)
- Ácido clorhídrico para ajustar el pH
- Hidróxido de sodio para ajustar el pH
- Agua para preparaciones inyectables
Aspecto del producto y contenido del envase
El producto es un gel intrauterino (dentro del útero) que es estéril, transparente o casi transparente, líquido viscoso ligeramente marrón-amarillento a temperatura ambiente, que contiene 42 mg/ml de lidocaína. La formulación muestra una gelificación reversible dependiente de la temperatura, es un gel a temperatura corporal (termogelificación). El gel intrauterino Lidbree 42 mg/ml se presenta en una jeringa precargada estéril de 10 ml (copolímero de olefina cíclica) con tapón de punta de goma de bromobutilo y tapón, acondicionado en un blister con la varilla del émbolo. La jeringa se gradúa en ml. Se proporciona un aplicador estéril (polipropileno) con un accesorio de bloqueo Luer compatible con la jeringa precargada en una bolsa separada dentro de la caja. Se pueden extraer 8,5 ml de gel del aplicador..
Tamaño del envase: 1 × 10 ml de gel intrauterino en jeringa precargada.
Símbolos de la etiqueta del aplicador de Lidbree
|
|
|
|
|
Número de catálogo | Código de lote | No utilizar si el envase está dañado | No reutilizar | Marcado CE |
|
|
|
| |
Fabricante | Fecha de caducidad | Estéril por irradiación | Consultar las instrucciones de uso |
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Gedeon Richter Plc.
Gyömroi út 19-21.
Budapest H-1103
Hungría
Fabricantes:
Recipharm Karlskoga AB
Björkbornsvägen 5
SE691 33 Karlskoga
Suecia
Gedeon Richter Plc.
Gyömroi út 19-21.
Budapest H-1103Hungría
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorizaciónde comercialización:
Gedeon Richter Ibérica, S.A.
Sabino Arana, 28 - 4º 2ª
08028 Barcelona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Bélgica, Bulgaria, Chipre, Chequia, Alemania, Dinamarca, Estonia, Grecia, España, Finlandia, Francia, Croacia, Hungría, Irlanda, Islandia, Italia, Luxemburgo, Letonia, Lithuania, Malta, Noruega, Polonia, Portugal, Reino Unido, Rumanía, Suecia, Eslovenia, Eslovaquia: Lidbree
Fecha de la última revisión de esteprospecto:Junio2020
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)(http://www.aemps.gob.es/)}>
----------------------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Uso exclusivo por vía endocervical e intrauterina.
Después del uso de Lidbree, en casos de inserción difícil y/o dolor excepcional o sangrado durante o después de la inserción, deberá realizarse inmediatamente una exploración física y una ecografía para descartar una perforación del cuerpo o del cuello uterino, ya que como sucede con una anestesia tópica efectiva el paciente podría no reaccionar con dolor en caso de perforación.
Formulación termogelificante:Lidbree es un anestésico local, líquido viscoso termogelificante, sin conservantes. La formulación forma un gel cuando la temperatura aumenta a la temperatura corporal quedando adherida al tejido mucoso del canal cervial y del útero (minimizando las pérdidas que se producirían con una formulación líquida).
Metodo de aplicación y dosis
Lidbree debe administrarse en estado líquido. Si se forma un gel, colocar en el refrigerador hasta que se vuelva líquido nuevamente. La burbuja de aire visible en la jeringa se mueve si la jeringa está inclinada.
Preparar el aplicador estéril incluido en el envase siguiendo los pasos indicados y aplicar el medicamento:
- Comprobar el aspecto de la jeringa mientras la inclina. La burbuja de aire se moverá al inclinar la jeringa cuando el producto está en estado líquido listo para usar. Si la burbuja de aire no se mueve, se debe a que se ha formado un gel, en ese caso deberá colocar la jeringa en el refrigerador hasta que se vuelva líquido de nuevo.
- Conectar la varilla del émbolo y el aplicador a la jeringa, asegurándose de que estén bien encajados.

- Extraer la burbuja de aire y llenar el aplicador con gel empujando con cuidado el émbolo de la jeringa.
- Use la escala de centímetros del aplicador para medir la cantidad de Lidbree.
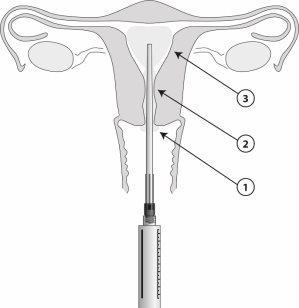
Una vez colocado el aplicador, se pueden administrar 8,5 ml de gel desde la jeringa. Un ml contiene 42 mg de lidocaína. Aplique el gel paso a paso (1 a 3) como se ilustra en la figura.
Procedimientos cervicales
- Utilizando el aplicador estéril, aplicar de 2 a 3 ml del gel en una capa gruesa en el exocervix.
- Utilizando el aplicador, administrar 3 ml en el canal cervical 5 minutos antes del inicio del procedimiento.
Procedimientos intrauterinos
- Utilizando el aplicador estéril, aplicar de 1 a 2 ml del gel en el labio anterior del exocervix.
- Utilizando el aplicador, administrar de 2 a 3 ml en el canal cervical. Esperar 2 minutos para que comience el efecto en el canal interno.
- Después, inserte el aplicador en la cavidad uterina e introduzca 3 a 5 ml del gel, 5 minutos antes del procedimiento. El aplicador está marcado con una escala en centímetros. Se puede administrar un volumen menor, p. ej. en pacientes nulíparas, si la paciente experimenta molestias antes de acabar administrar todo el volumen.
Una dosis intrauterina única no debe exceder los 10 ml. Deseche el contenido no utilizado.
Población pediátrica a partir de 15 años.
En adolescentes con bajo peso, peso corporal por debajo de 30 kg, la dosis debe reducirse proporcionalmente, la dosis única no debe exceder la dosis parenteral máxima recomendada (6 mg/kg de clorhidrato de lidocaína, que corresponde a 5,2 mg/kg de lidocaína base en Lidbree, es decir, 1,2 ml por 10 kg de peso corporal). En adolescentes con un peso corporal de 30 kg, la dosis máxima de Lidbree es de 3,6 ml en total.
Duración del efecto
Se ha demostrado que el gel reduce el dolor durante los procedimientos ginecológicos y hasta al menos 30 minutos después del procedimiento. Después de 1 hora, el efecto analgésico ha desaparecido.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LIDBREE 42 MG/ML GEL INTRAUTERINOForma farmacéutica: APÓSITO, 700 mgPrincipio activo: LidocainaFabricante: Grünenthal Pharma S.A.Requiere recetaForma farmacéutica: GEL/PASTA/LIQUIDO BUCAL, 20 mg/gPrincipio activo: LidocainaFabricante: Chemische Fabrik Kreussler & Co GmbhNo requiere recetaForma farmacéutica: GEL, 20 mg/mlPrincipio activo: LidocainaFabricante: Farco-Pharma GmbhRequiere receta
Médicos online para LIDBREE 42 MG/ML GEL INTRAUTERINO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LIDBREE 42 MG/ML GEL INTRAUTERINO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes