
LIBMELDY 2-10 X 10^6 CELULAS/ML DISPERSION PARA PERFUSION

Cómo usar LIBMELDY 2-10 X 10^6 CELULAS/ML DISPERSION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente o cuidador
Libmeldy 2-10 x 106células/ml dispersión para perfusión
atidarsagén autotemcel
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que su hijo pudiera tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de que se le administre este medicamento a su hijo, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte al médico o enfermero de su hijo.
- Si su hijo experimenta efectos adversos, consulte al médico o enfermero de su hijo, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver la sección 4.
- El médico o enfermero de su hijo le entregará una tarjeta de información para el paciente que contiene información de seguridad importante que necesita conocer sobre el tratamiento de su hijo con Libmeldy. Lea la tarjeta de información para el paciente atentamente y siga las instrucciones que contiene.
- Debe llevar consigo la tarjeta de información para el paciente con usted, en todo momento, y mostrársela al médico o enfermero cuando los vea o si ingresan a su hijo en el hospital.
Contenido del prospecto
- Qué es Libmeldy y para qué se utiliza
- Qué necesita saber antes de que se le administre Libmeldy a su hijo
- Cómo se elabora y administra Libmeldy
- Posibles efectos adversos
Efectos adversos del medicamento de acondicionamiento
Efectos adversos de Libmeldy
- Conservación de Libmeldy
- Contenido del envase e información adicional
1. Qué es Libmeldy y para qué se utiliza
Qué es Libmeldy
Libmeldy es un tipo de medicamento llamado terapia génica. Está elaborado especialmente para su hijo a partir de su propia médula ósea o células sanguíneas.
Para qué se utiliza Libmeldy
Libmeldy se utiliza para tratar una enfermedad grave llamada leucodistrofia metacromática (LDM):
- en niños con las formas «infantil tardía» o «juvenil temprana» de la enfermedad que aún no han desarrollado ningún signo o síntoma;
- en niños con la forma «juvenil temprana» de la enfermedad que han empezado a desarrollar síntomas, pero cuyos síntomas aún no empeoran rápidamente.
Las personas con LDM presentan un fallo en el gen para producir una enzima llamada arilsulfatasa A (ARSA). Esto conduce a una acumulación de sustancias llamadas sulfatos en el cerebro y en el sistema nervioso, lo que causa daños en el sistema nervioso y una pérdida progresiva de capacidades físicas y, más tarde, de capacidades mentales, lo que en última instancia conduce a la muerte.
¿Cómo actúa Libmeldy?
Las células llamadas células madre se obtienen de la médula ósea o de la sangre de su hijo. Luego se modifican en un laboratorio para introducir un gen funcional para obtener ARSA. Cuando se le suministra a su hijo Libmeldy, que está compuesto por estas células modificadas, las células comenzarán a producir ARSA para descomponer los sulfatos de las células nerviosas y otras células del cuerpo de su hijo. Se espera que este proceso frene la progresión de la enfermedad y mejore la calidad de vida de su hijo.
Libmeldy se administra por goteo (perfusión) en una vena (por vía intravenosa). Para obtener más información sobre lo que sucede antes y durante el tratamiento, ver la sección 3, Cómo se administra Libmeldy.
Si tiene alguna pregunta sobre cómo funciona Libmeldy o por qué se le ha recetado este medicamento a su hijo, pregunte al médico de su hijo.
2. Qué necesita saber antes de que se le administre Libmeldy a su hijo
No se debe administrar Libmeldy a su hijo:
- Si su hijo es alérgico a alguno de los componentes de este medicamento (incluidos en la sección 6). Si cree que su hijo puede ser alérgico, pregunte a su médico.
- Si su hijo se ha sometido previamente a una terapia génica con sus células madre sanguíneas.
- Si su hijo es alérgico/a o si su médico piensa que su hijo podría experimentar efectos adversos inaceptables por cualquiera de los ingredientes de los medicamentos que su hijo recibirá antes del tratamiento con Libmeldy (ver la sección 3).
Advertencias y precauciones
- La información sobre los medicamentos basados en células como Libmeldy se debe conservar durante 30 años en el hospital. La información que se guardará sobre su hijo será su nombre y el número de lote de Libmeldy que ha recibido.
- Libmeldy se obtiene de las células madre de su hijo y solo se le debe administrar a su hijo.
Antes del tratamiento con Libmeldy
- La evaluación de su hijo por su médico para confirmar que tiene LDM y evaluar los síntomas y efectos de su enfermedad se llevará a cabo antes de tomar la decisión de usar Libmeldy. Es posible que su hijo no muestre ningún signo físico de la enfermedad en el momento de la evaluación inicial.
Si la LDM de su hijo ha progresado y ha empeorado antes del inicio del tratamiento, su médico puede determinar que su enfermedad ha alcanzado una «fase de rápida progresión». Si esto sucede, es posible que su hijo no pueda beneficiarse del tratamiento y que el médico de su hijo decida no administrarle Libmeldy.
- Es posible que su hijo reciba medicamentos conocidos como medicamentos de movilizacióny acondicionamiento(consulte las secciones 3 y 4 para obtener más información sobre estos medicamentos, incluidos los posibles efectos adversos).
- Los catéteres venosos centrales son tubos delgados y flexibles que el médico introduce en una vena grande para acceder al torrente sanguíneo de su hijo. Los riesgos de estas vías son las infecciones y la formación de coágulos de sangre. El médico y los enfermeros vigilarán a su hijo para detectar cualquier complicación del catéter venoso central.
- Libmeldy se ha sometido a pruebas para detectar la presencia de microorganismos infecciosos antes de ser administrado a su hijo. Existe un pequeño riesgo de infección. Los médicos y los enfermeros de su hijo realizarán un seguimiento durante toda la perfusión para detectar signos de infección y proporcionar tratamiento si fuera necesario.
- El médico revisará la glándula tiroides de su hijo. La glándula tiroides se encuentra en el cuello y produce hormonas que son importantes para ayudar al cuerpo a funcionar con normalidad. También se realizará un seguimiento después del tratamiento si se requiere.
Después del tratamiento con Libmeldy
- Después del tratamiento, es posible que se le pida a su hijo que se inscriba en un estudio de seguimientodurante un máximo de 15 años para comprender mejor los efectos a largo plazo de Libmeldy.
- Si su hijo requiere una transfusión de sangre dentro de los primeros tres meses después de haber recibido Libmeldy, los productos sanguíneos deben ser irradiados. Esto significa que los glóbulos blancos, llamados linfocitos, se han reducido para minimizar el riesgo de una reacción a la transfusión. El médico supervisará a su hijo para detectar cualquier accidente transfusional.
- Los glóbulos rojos de su hijo estarán bajos durante algún tiempo después del tratamiento con Libmeldy. Esto afecta a las células sanguíneas que luchan contra las infecciones, llamadas neutrófilos, y que se pueden medir con un simple análisis de sangre. Si los neutrófilos de su hijo siguen siendo bajos después de 60 días, esto podría denominarse «fallo del injerto». En tal caso, el médico de su hijo puede decidir introducir las células de rescate previamente extraídas (ver la sección 3). A las células de rescate no se les ha añadido el gen funcional ARSA y no producirán tal enzima.
- Después de recibir el medicamento de acondicionamiento, su hijo puede tener un número bajo de plaquetas en sangre. Esto significa que es posible que la sangre de su hijo no pueda coagularse con normalidad y que su hijo sea propenso a las hemorragias durante algún tiempo después del tratamiento. El médico controlará el recuento de plaquetas de su hijo con análisis de sangre y le proporcionará un tratamiento si se requiere. Esto puede incluir una transfusión de plaquetas para ayudar a aumentar su recuento de plaquetas.
- Puede producirse una acidosis metabólica. Es una condición en la que el nivel de ácido en la sangre se eleva. Puede haber muchas razones diferentes para que se produzca, y es más común en pacientes con LDM. Los síntomas de la acidosis metabólica incluyen la sensación de falta de aliento, respiración rápida, náusea (sensación de malestar) y vómitos. El médico supervisará a su hijo para detectar signos y síntomas de acidosis metabólica.
- La inserción de un nuevo gen en las células madre podría en teoría causar cáncer en la sangre (leucemia y linfoma). Después del tratamiento, su médico supervisará a su hijo para detectar cualquier signo de leucemia o linfoma.
- Durante los estudios clínicos, algunos pacientes desarrollaron anticuerpos contra la enzima ARSA, llamados anticuerpos contra ARSA (ver los efectos adversos de Libmeldy en la sección 4). Esto se resolvió por sí solo o después del tratamiento con medicamentos adaptados. El médico de su hijo analizará su sangre en busca de anticuerpos contra la ARSA y le proporcionará un tratamiento si se requiere.
- Después de que su hijo haya recibido Libmeldy, se le supervisará con análisis de sangre regulares. Esto incluirá la medición de anticuerpos, conocidos como inmunoglobulinas, en su sangre. Si el nivel es bajo, su hijo puede requerir un tratamiento de sustitución con inmunoglobulina. El médico de su hijo hablará con usted si se requiere.
- Libmeldy se obtiene utilizando partes del virus de la inmunodeficiencia humana (VIH), que han sido alteradas para que no puedan causar una infección. El virus alterado se utiliza para insertar el gen ARSA en las células madre de su hijo. Aunque este medicamento no infectará con VIH a su hijo, tener Libmeldy en su sangre puede causar un falso positivo en la prueba del VIH con algunas pruebas comerciales (las llamadas «pruebas basadas en RCP») que reconocen una parte del VIH utilizada para elaborar Libmeldy. Si el resultado de la prueba de VIH de su hijo es positivo después del tratamiento con Libmeldy, contacte con el médico o enfermero de su hijo.
- Después de un tratamiento con Libmeldy, su hijo no podrá donar sangre, órganos, tejidos ni células. Esto se debe a que Libmeldy es un producto de terapia génica.
Antes de que se suministre Libmeldy a su hijo, el médico:
- Revisará los pulmones, el corazón, los riñones, el hígado y la presión arterial de su hijo.
- Buscará señales de infección; se tratará cualquier infección antes de que su hijo reciba Libmeldy.
- Comprobará la presencia de hepatitis B, hepatitis C, virus de las leucemias de las células T (HTLV), VIH o infección por micoplasma.
- Comprobará si su hijo ha sido vacunado en las seis semanas anteriores o si está prevista una
- vacunación en los próximos meses.
Cuando no se puede completar el tratamiento de Libmeldy
Antes de que se le administre Libmeldy, su hijo recibirá un medicamento de acondicionamiento para eliminar las células de su médula ósea.
Si no se puede administrar Libmeldy después de que su hijo haya recibido el medicamento de acondicionamiento, o si las células madre modificadas no se fijan (injertan) en el cuerpo de su hijo, el médico puede decidir devolver las células de rescate previamente extraídas a su hijo por medio de una perfusión (ver también la sección 3, Cómo se administra Libmeldy). A las células de rescate no se les ha añadido el gen funcional ARSA y no producirán tal enzima. Para obtener más información, póngase en contacto con el médico de su hijo.
Otros medicamentos y Libmeldy
Informe a su médicosi su hijo está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento, incluidos los que se pueden adquirir sin receta.
- Su hijo no debe tomar ningún medicamento para la infección por VIH desde al menos un mes antes de que se le administren los medicamentos de movilización o se le tome una muestra de médula ósea, hasta al menos siete días después de la perfusión con Libmeldy (ver también la sección 3, Cómo se elabora y se administra Libmeldy).
- No se debe administrar a su hijo vacunas llamadas vacunas con virus vivos atenuadosdurante seis semanas antes de que se le administre el medicamento de acondicionamiento para prepararlo para el tratamiento de Libmeldy, ni después del tratamiento mientras el sistema inmunológico de su hijo (el sistema de defensa del cuerpo) se esté recuperando.
Conducción y uso de máquinas
La influencia de Libmeldy sobre la capacidad para conducir o utilizar máquinas es nula. Sin embargo, los medicamentos de movilización y acondicionamiento pueden causar mareos y fatiga.
Libmeldy contiene sodio y dimetilsulfóxido
Este medicamento contiene 35-560 mg de sodio (principal componente de la sal común/de mesa) en cada dosis. Esto equivale al 2-28 % de la ingesta diaria máxima de sodio recomendada para un adulto.
Si su hijo no ha estado en contacto anteriormente con dimetilsulfóxido (una sustancia utilizada para preservar las células congeladas), el médico o enfermero deben supervisar a su hijo para detectar cualquier reacción durante la perfusión y a cada hora durante las tres horas siguientes a la perfusión.
3. Cómo se elabora y administra Libmeldy
Dado que Libmeldy se obtiene de las propias células madre de su hijo, se extraerá la médula ósea o la sangre de su hijo para preparar el medicamento unos dos meses antes del tratamiento. La médula ósea se puede extraer de los huesos de la cadera de su hijo y la sangre se puede extraer de las venas de su hijo. Para obtener más información, pregunte a su médico.
Si las células madre se obtienen de la médula ósea de su hijo:
- Su hijo recibirá medicación para que pueda relajarse y evitar el dolor o para que esté inconsciente antes del procedimiento. El médico extraerá la médula ósea de su hijo con una jeringa especial.
Si las células madre se obtienen de la sangre de su hijo:
- Primero se le administrará a su hijo un medicamento de movilización para trasladar las células madre sanguíneas de la médula ósea a la corriente sanguínea.
- Entonces, las células madre sanguíneas se pueden extraer con una máquina que separa los componentes de la sangre (máquina de aféresis). Puede llevar más de un día extraer suficientes células madre de la sangre para producir Libmeldy.
Las células madre extraídas de la médula ósea o de la sangre se dividirán en:
- La muestra de reserva, que se congelará y almacenará, se le administrará a su hijo como células madre de sustitución si Libmeldy no se puede administrar o no funciona (ver «Cuando no se puede completar el tratamiento de Libmeldy» en la sección 2).
- La muestra del tratamiento, que se enviará para preparar Libmeldy, introduciendo una copia del gen funcional ARSA en las células madre de la muestra.
Cómo se administra Libmeldy a su hijo
- Libmeldy se le suministrará a su hijo en un centro de tratamiento especializado y por médicos especializados en el uso de este tipo de medicina.
- Los médicos comprobarán que las bolsas de perfusión de Libmeldy están identificadas como elaboradas con la propia muestra de su hijo.
- Libmeldy es un tratamiento que solo se puede utilizar una vez. No se podrá administrar de nuevo a su hijo en el futuro.
Cuándo | Qué sucede | Por qué |
De manera aproximada dos meses antes de la perfusión de Libmeldy | Se administra el medicamento de movilización si Libmeldy se ha elaborado a partir de células madre sanguíneas | Para trasladar las células madre sanguíneas de la médula ósea de su hijo a la corriente sanguínea. |
De manera aproximada dos meses antes de la perfusión de Libmeldy | Se extrae sangre o médula ósea | Para elaborar Libmeldy y para servir como células de sustitución en caso necesario |
Cinco días antes de la perfusión de Libmeldy | Se administrará un medicamento de acondicionamiento durante 3- 4 días en un hospital | Para preparar la médula ósea de su hijo para el tratamiento destruyendo las células de la médula ósea de forma que puedan ser sustituidas por las células modificadas de Libmeldy. |
De 15 a 30 minutos antes de la perfusión de Libmeldy | Se podrá administrar un medicamento llamado antihistamínico | Para ayudar a prevenir una respuesta alérgica a la perfusión. |
Comienzo de la perfusión con Libmeldy | Libmeldy se administra por goteo (perfusión) en una vena. Esto se llevará a cabo en un | Para añadir células madre sanguíneas con el gen ARSA en la médula ósea de su hijo. |
hospital y tardará menos de 30 minutos para cada bolsa de perfusión. El número de bolsas variará en función del paciente. | ||
Después de la perfusión de Libmeldy | Su hijo permanecerá en el hospital durante 4-12 semanas, aproximadamente | Para recuperarse y que le supervisen a fin de comprobar si el tratamiento de su hijo está funcionando y actuar si aparece algún efecto adverso hasta que el médico considere que es seguro para su hijo salir del hospital. |
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos están relacionados con el medicamento de acondicionamiento utilizado para preparar la médula ósea de su hijo para el tratamiento con Libmeldy.
Hable con el médico de su hijo sobre los efectos adversos del medicamento de acondicionamiento.
También puede leer los prospectos de ese medicamento.
Efectos adversos del medicamento de acondicionamiento
Avise inmediatamente a su médico o enfermerosi su hijo sufre alguno de los siguientes efectos adversos después de recibir el medicamento de acondicionamiento. Suelen aparecer entre los primeros días y varias semanas después de recibir el medicamento de acondicionamiento, pero también pueden desarrollarse mucho más tarde.
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- análisis de sangre que muestra un bajo nivel de glóbulos blancos con o sin fiebre
- acidosis metabólica, una condición en la que los niveles de ácido en la sangre son elevados
- inflamación y llagas en la boca y los labios
- malestar (vómitos)
- hepatomegalia
- dolor en la parte superior derecha del abdomen (vientre) debajo de las costillas, coloración amarillenta de los ojos o la piel, rápido aumento de peso, hinchazón de los brazos, las piernas y el abdomen, y dificultad para respirar. Estos pueden ser signos de una enfermedad hepática grave denominada enfermedad venooclusiva
- pérdida o disminución de la función de los ovarios
Efectos adversos frecuentes (pueden afectar a hasta 1 de cada 10 personas)
- hemorragias o hematomas anómalos: pueden estar causados por un nivel bajo de plaquetas, lo que reduce la capacidad de coagulación de la sangre
- infecciones que podrían provocar a su hijo sensación de calor (fiebre), de frío o sudor
- infección en el pecho (neumonía)
- infección de los órganos implicados en la excreción de orina (como la vejiga y el tracto urinario)
- número bajo de glóbulos rojos (anemia)
- exceso de líquido en el cuerpo
- acumulación de líquido en el abdomen
- problemas para dormir
- cefalea
- hemorragias nasales
- dolor en la boca y la garganta
- diarrea
- hemorragias en el tracto intestinal
- malestar (náusea)
- aumento de las enzimas hepáticas (transaminasas y aminotransferasas) observadas en los análisis de sangre
- prurito
- dolor de espalda
- dolor óseo
- disminución de la producción de orina
- fiebre
- prueba de Aspergilluspositiva (enfermedad pulmonar causada por un hongo)
Efectos adversos de Libmeldy
Se han notificado los siguientes efectos adversos con Libmeldy.
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- prueba positiva de anticuerpos contra ARSA. Los anticuerpos son la defensa natural del cuerpo contra cualquier elemento que el cuerpo considere como extraño.
Comunicación de efectos adversos
Si su hijo experimenta efectos adversos, consulte al médico o enfermero de su hijo, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Libmeldy
Esta información está dirigida solo a los médicos.
Dado que este medicamento se administrará en un hospital, este será responsable de la correcta conservación del medicamento antes y durante su uso, así como de su correcta eliminación.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase exterior y las etiquetas de la bolsa de perfusión.
No utilice este medicamento si la bolsa de perfusión está dañada o tiene fugas.
Almacenar a <-130 ºC hasta un máximo de seis meses. No descongelar el medicamento hasta que esté listo para utilizarse. Una vez descongelado, conservar a temperatura ambiente (20 ºC-25 ºC) y usar en un plazo de dos horas. No volver a congelar.
Este medicamento contiene células humanas genéticamente modificadas. El medicamento que no se utilice o el material de desecho deben eliminarse de conformidad con las directrices locales sobre la manipulación de materiales de origen humano.
6. Contenido del envase e información adicional
Composición de Libmeldy
El principio activo de Libmeldy consiste en las propias células madre de su hijo que contienen copias funcionales del gen ARSA. La concentración por bolsa es de 2-10 × 106 células por mililitro.
Los demás ingredientes son una solución utilizada para preservar las células congeladas y el cloruro de sodio (ver la sección 2, Libmeldy contiene sodio).
Aspecto de Libmeldy y contenido del envase
Libmeldy es una dispersión de células color transparente a ligeramente turbio, de incoloro a amarillo o rosado, que se suministra en una o más bolsas de perfusión transparentes, cada una de ellas empaquetada en una bolsa dentro de un contenedor cerrado.
El nombre y la fecha de nacimiento de su hijo, así como la información codificada que identifica a su hijo como paciente, están impresos en cada bolsa de perfusión y en cada contenedor.
Titular de la autorización de comercialización
Orchard Therapeutics (Netherlands) B.V.
Basisweg 10,
1043AP Ámsterdam,
Países Bajos
Responsable de la fabricación
AGC Biologics S.p.A.
Zambon Scientific Park
Via Meucci 3
200091 Bresso (MI)
Italia
AGC Biologics S.p.A.
Via Olgettina 58
20132
Milán
Italia
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
< ---------------------------------------------------------------------------------------------------------------------------- >
Esta información está destinada únicamente a profesionales sanitarios:
Es importante que lea todo el contenido de este procedimiento antes de administrar Libmeldy.
Precauciones que se deben tomar antes de manipular o administrar el medicamento
- Este medicamento contiene glóbulos rojos humanos genéticamente modificados. Los profesionales sanitarios que manipulen Libmeldy deben tomar las precauciones adecuadas (usar guantes, ropa de protección y protección ocular) para evitar la posible transmisión de enfermedades infecciosas.
- Libmeldy debe permanecer a <-130 ºC en todo momento, hasta que el contenido de la bolsa se descongele para la perfusión.
Definición de la dosis administrada
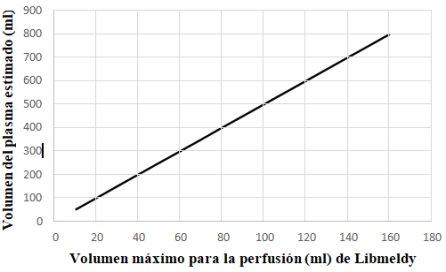
- La dosis que se va a perfundir y el número de bolsas de perfusión de Libmeldy que se van a utilizar deben definirse según el número total de células CD34+ suministradas que se indica en la etiqueta de información del lote (es decir, la «dosis suministrada», calculada según el peso del paciente en el momento de la extracción de las células). La dosis de Libmeldy que se administre también debe tener en cuenta el peso del paciente en el momento del tratamiento, y el hecho de que cualquier bolsa que se utilice se debe administrar en su totalidad.
- Se debe tener una especial consideración del volumen de la perfusión en relación con la edad y el peso del paciente. Cuando la dosis de perfusión de Libmeldy deba ser de más de una bolsa, se debe asegurar antes de la perfusión que el volumen del medicamento que se va a perfundir es compatible con el límite recomendado, es decir, el volumen total de dimetilsulfóxido administrado debe ser <1 % del volumen del plasma estimado del paciente. Por lo tanto, el volumen máximo de Libmeldy administrado debe ser <20 % del volumen del plasma estimado del paciente.
- El siguiente gráfico se proporciona como referencia para determinar el volumen máximo de Libmeldy que se puede perfundir a un paciente según su volumen del plasma estimado.
Directriz sobre el límite de seguridad de dimetilsulfóxido: el volumen máximo de administración de Libmeldy debe ser < 20 % del volumen del plasma estimado del paciente.
Preparación de la perfusión
- Se pueden utilizar varias bolsas de perfusión para cada paciente. Cada bolsa de perfusión se proporciona dentro de una bolsa de envoltura completa que se encuentra dentro de un cartucho metálico.
- La(s) bolsa(s) de perfusión envuelta(s) debe(n) mantenerse dentro de lo(s) cartucho(s) metálico(s) en la fase de vapor de nitrógeno líquido a <-130 ºC hasta que esté(n) lista(s) para la descongelación y perfusión.
- Haga un recuento de todas las bolsas de perfusión y confirme que cada bolsa de perfusión está dentro de la fecha de caducidad indicada en la etiqueta de información del lote adjunta.
- Se debe disponer de una solución inyectable estéril de cloruro de sodio de 9 mg/ml (0,9 %), para imprimar el tubo antes de la perfusión y para lavar la bolsa y el tubo después de la perfusión.
Comprobación antes de la descongelación
- No saque el cartucho metálico del almacenamiento criogénico ni descongele a Libmeldy hasta que el paciente esté listo para la perfusión. Se debe coordinar el momento de descongelación de la(s) bolsa(s) de perfusión que contiene(n) Libmeldy y de la perfusión. Confirme el tiempo de perfusión con antelación y ajuste el tiempo de inicio con la descongelación de manera que Libmeldy esté disponible para la perfusión cuando el perceptor esté listo.
- Abra el cartucho metálico e inspeccione la bolsa de envoltura completa y la bolsa de perfusión para detectar cualquier pérdida de integridad antes de la descongelación. Si una bolsa de perfusión está afectada, siga las pautas locales para la manipulación de residuos materiales de origen humano y póngase en contacto con Orchard Therapeutics inmediatamente.
- Antes de descongelar Libmeldy, se debe verificar que la identidad del paciente coincida con la información exclusiva del paciente que figura en las etiquetas de los envases y en la etiqueta de información del lote que se incluye. Libmeldy está destinado exclusivamente a un uso autógeno. No descongele ni perfunda Libmeldy si la información de la etiqueta específica del paciente en la bolsa de perfusión no coincide con el paciente.
Descongelación
- Después de extraerla cuidadosamente del cartucho metálico, descongele la bolsa de perfusión en su bolsa de envoltura completa hermética a 37 ºC en un material de descongelación controlado hasta que no haya hielo visible en la bolsa de perfusión.
- Una vez que haya finalizado la descongelación, la bolsa se debe extraer inmediatamente del material de descongelación.
- La bolsa de envoltura completa se debe abrir cuidadosamente para extraer la bolsa de perfusión, que se debe mantener a temperatura ambiente (20 ºC-25 ºC) hasta la perfusión.
- Masajee suavemente la bolsa de perfusión para volver a suspender las células. Se debe inspeccionar el contenido de la bolsa de perfusión en busca de cualquier agregado celular visible restante. Los pequeños grupos de material celular deben dispersarse con un mezclado manual suave. No agite la bolsa.
- La bolsa de perfusión no se debe lavar, centrifugar, tomar como muestra ni volver a suspender en otros medios antes de la perfusión.
- No se debe irradiar Libmeldy, ya que la irradiación podría provocar la inactivación del producto.
- Si se proporciona más de una bolsa de perfusión para la dosis de tratamiento del paciente, la siguiente bolsa solo se debe descongelar después de que el contenido de la bolsa anterior se haya perfundido completamente.
Administración
- Libmeldy debe administrarse como una perfusión intravenosa a través de un catéter venoso central, de acuerdo con los procedimientos estándar del centro de tratamiento especializado en la administración de los productos de terapia celular.
- El equipo de administración recomendado consiste en un transfusor de sangre equipado con un filtro de 200 µm.
- Cada bolsa se debe perfundir por gravedad en las dos horas siguientes a la descongelación, incluida cualquier interrupción durante la perfusión, para mantener la máxima viabilidad del producto.
- La velocidad máxima de perfusión es de 5 ml/kg/h, y el contenido de cada bolsa se debe perfundir en aproximadamente 30 minutos.
- Cuando necesite más de una bolsa de Libmeldy, solo debe perfundir una bolsa del producto por hora.
- Los pacientes que no hayan estado expuestos previamente al dimetilsulfóxido se deben someter a un seguimiento exhaustivo. Se deben controlar los signos vitales (presión sanguínea, frecuencia cardíaca y saturación de oxígeno) y la aparición de cualquier síntoma durante un máximo de tres horas después de la perfusión.
- Al final de la perfusión, enjuague los restos de Libmeldy que queden en la bolsa de perfusión y cualquier tubo utilizado con la solución inyectable de cloruro de sodio de 9 mg/ml (0,9 %) para asegurar que se perfunda al paciente la mayor cantidad de células posible. Se debe tener una especial consideración del volumen de la perfusión en relación con la edad y el peso del paciente.
Precauciones que se deben tomar en la eliminación del medicamento
- Libmeldy contiene células humanas genéticamente modificadas. Deberán seguirse las orientaciones locales sobre la manipulación de material de origen humano en el caso de los medicamentos no utilizados o de material de desecho.
- Todo el material que haya estado en contacto con Libmeldy (residuos sólidos y líquidos) se debe manipular y eliminar como residuos potencialmente infecciosos de conformidad con las orientaciones locales sobre la manipulación de material de origen humano.
Exposición accidental
- Se debe evitar la exposición accidental a Libmeldy. En caso de exposición accidental, deben seguirse las orientaciones locales sobre la manipulación de materiales de origen humano, que pueden incluir el lavado de la piel contaminada y la eliminación de la ropa contaminada. Las superficies de trabajo y los materiales que hayan estado potencialmente en contacto con Libmeldy se deben descontaminar con un desinfectante adecuado.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LIBMELDY 2-10 X 10^6 CELULAS/ML DISPERSION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 100 UPrincipio activo: LaronidasaFabricante: Sanofi B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 30 mg/mlPrincipio activo: Cerliponasa alfaFabricante: Biomarin International LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, DesconocidaPrincipio activo: ImiglucerasaFabricante: Sanofi B.V.Requiere receta
Médicos online para LIBMELDY 2-10 X 10^6 CELULAS/ML DISPERSION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LIBMELDY 2-10 X 10^6 CELULAS/ML DISPERSION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















