
BONDRONAT 6 mg CONCENTRADO PARA SOLUCION PARA PERFUSION

Cómo usar BONDRONAT 6 mg CONCENTRADO PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Bondronat 6 mg concentrado para solución para perfusión
ácido ibandrónico
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4
Contenido del prospecto:
- Qué es Bondronat y para qué se utiliza
- Qué necesita saber antes de empezar a recibir Bondronat
- Cómo recibir Bondronat
- Posibles efectos adversos
- Conservación de Bondronat
- Contenido del envase e información adicional
1. Qué es Bondronat y para qué se utiliza
Bondronat contiene como principio activo ácido ibandrónico. Éste pertenece a un grupo de medicamentos llamado bifosfonatos.
Bondronat se usa en adultos y se le ha recetado Bondronat si tiene cáncer de mama que se ha extendido hasta los huesos (llamado ‘metástasis óseas’)
- Le ayuda a prevenir que sus huesos se rompan (fracturas)
- Le ayuda a prevenir otros problemas óseos que pudieran necesitar cirugía o radioterapia
También se le puede recetar Bondronat si tiene un nivel de calcio en sangre elevado debido a un tumor.
Bondronat actúa reduciendo la cantidad de calcio que se pierde de sus huesos. Esto ayuda a frenar que sus huesos se hagan más débiles.
2. Qué necesita saber antes de empezar a recibir Bondronat
No reciba Bondronat:
- si es alérgico al ácido ibandrónico o a cualquiera de los demás componentes de este medicamento que se mencionan en la sección 6
- si tiene o ha tenido niveles bajos de calcio en sangre
No reciba este medicamento si le pasa algo de lo mencionado arriba. Si no está seguro, consulte a su médico o farmacéutico antes de recibir Bondronat.
Advertencias y precauciones
Se ha comunicado de forma muy rara una reacción adversa denominada osteonecrosis de la mandíbula (ONM) (daño óseo en la mandíbula) durante la experiencia poscomercialización en pacientes tratados con Bondronat para trastornos relacionados con el cáncer. La ONM también puede aparecer tras interrumpir el tratamiento.
Es importante tratar de prevenir el desarrollo de ONM ya que es un estado doloroso que puede ser difícil de tratar. Con el fin de reducir el riesgo de desarrollar osteonecrosis de la mandíbula, se deben tomar ciertas precauciones.
Antes de recibir el tratamiento, informe a su médico/enfermero (profesional sanitario) si:
- tiene problemas en la boca o dientes, como son, una salud dental pobre, enfermedad de las encías, o una extracción de los dientes planificada
- no recibe un cuidado dental rutinario o si no ha tenido una revisión dental desde hace mucho tiempo
- es fumador (ya que esto puede incrementar el riesgo de problemas dentales)
- ha sido tratado previamente con un bifosfonato (utilizado para tratar o prevenir alteraciones óseas)
- está tomando medicamentos denominados corticosteroides (tales como prednisolona o dexametasona)
- tiene cáncer.
Su médico le puede pedir que se someta a un examen dental antes de iniciar el tratamiento con Bondronat.
Mientras esté en tratamiento, debe mantener una buena higiene bucal (incluyendo cepillado regular de los dientes) y someterse a revisiones dentales rutinarias. Si lleva dentadura postiza, debe asegurarse que esté fijada adecuadamente. Si está bajo tratamiento dental o va a someterse a una cirugía dental (p. ej. extracción dental), informe a su médico acerca de su tratamiento dental e informe a su dentista que esté en tratamiento con Bondronat.
Contacte con su médico y su dentista inmediatamente si experimenta cualquier problema en la boca o dientes, tales como, pérdida dental, dolor o hinchazón, o dificultad en la curación de las úlceras o secreción, ya que estos pueden ser signos de osteonecrosis de la mandíbula.
Consulte a su médico, farmacéutico o enfermero antes de tomar Bondronat:
- si es alérgico a cualquier otro bifosfonato
- si tiene niveles altos o bajos de vitamina D, calcio o de cualquier otro mineral
- si tiene problemas de riñon
- si tiene problemas de corazón y su médico le ha recomendado limitar la ingesta diaria de líquidos
Se han observado casos graves, algunas veces mortales de reacción alérgica, en pacientes tratados con ácido ibandrónico intravenoso.
Debería avisar inmediatamente a su médico o enfermero si experimenta uno de los siguientes síntomas, como falta de aire/dificultad respiratoria, sensación de opresión en la garganta, hinchazón de la lengua, mareo, sensación de pérdida del conocimiento, rojez o hinchazón de la cara, sarpullido corporal, náuseas y vómitos (ver sección 4).
Niños y adolescentes
No se debe usar Bondronat en niños y adolescentes menores de 18 años.
Uso de Bondronat con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento. Esto es porque Bondronat puede afectar a la forma en la que otros medicamentos actúan. También otros medicamentos pueden afectar la forma en la que Bondronat actúa.
En concreto, informe a su médico o farmacéuticosi está recibiendo un tipo de antibiótico inyectado denominado ‘aminoglucósido’ como la gentamicina. Esto es porque tanto los aminoglucósidos como Bondronat pueden disminuirle la cantidad de calcio en sangre.
Embarazo y lactancia
No reciba Bondronat si está embarazada, planeando quedarse embarazada o si está en periodo de lactancia.
Consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Puede conducir y utilizar máquinas ya que se espera que Bondronat no tenga efecto o éste sea despreciable sobre su capacidad para conducir y utilizar máquinas. Informe primero a su médico si quiere conducir, utilizar máquinas o herramientas.
Bondronat contiene menos de 1 mmol de sodio (23 mg) por vial, es decir, está esencialmente ‘exento de sodio’.
3. Cómo recibir Bondronat
Administración de este medicamento
- Bondronat es normalmente administrado por un médico u otro personal sanitario con experiencia en el tratamiento del cáncer.
- Se administra mediante perfusión dentro de la vena
Su médico podría hacerle análisis de sangre periódicos mientras está recibiendo Bondronat. Esto es para comprobar que está recibiendo la cantidad correcta de este medicamento.
Cantidad que debe recibir
Su médico determinará la cantidad de Bondronat que le administrará dependiendo de su enfermedad. Si tiene un cáncer de mama que se ha extendido hasta los huesos, la dosis recomendada es de 3 viales (6 mg) cada 3-4 semanas, administrados mediante perfusión dentro de la vena durante al menos 15 minutos.
Si tiene un nivel de calcio en sangre elevado debido a un tumor, la dosis recomendada es una administración única de 2 mg ó 4 mg dependiendo de la gravedad de su enfermedad. Se debe administrar el medicamento mediante perfusión dentro de la vena durante dos horas. Se puede considerar repetir con otra dosis en caso de una respuesta insuficiente o si su enfermedad vuelve a aparecer.
Si tiene problemas de riñón, su médico ajustará la dosis y duración de la perfusión intravenosa.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico o enfermero inmediatamente si nota cualquiera de los siguientes efectos adversos graves ya que podría necesitar tratamiento médico urgente:
Raros(pueden afectar hasta 1 de cada 1.000 personas)
- dolor de ojo persistente e inflamación
- dolor nuevo, debilidad o molestias en el muslo, la cadera o la ingle. Pueden ser síntomas precoces de una posible fractura inusual del hueso del muslo
Muy raros(puede afectar hasta1 de cada 10.000 personas)
dolor o sensación de dolor en la boca o mandíbula. Pueden ser síntomas precoces de problemas graves de mandíbula [necrosis (muerte del tejido óseo) del hueso de la mandíbula]
- Consulte a su médico si usted tiene dolor de oído, el oído le supura o sufre una infección de oído. Estos podrían ser síntomas de daño en los huesos del oído.
- picor, hinchazón de la cara, labios, lengua y garganta, con dificultad para respirar. Puede que esté teniendo una reacción alérgica grave al medicamento (ver sección 2)
- reacciones adversas graves en la piel
De frecuencia no conocida (no se puede estimar la frecuencia con los datos disponibles)
- ataque de asma
Otros efectos adversos posibles
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- síntomas tipo gripal, incluyendo fiebre, escalofríos y tiritona, sensación de malestar, fatiga, dolor de huesos, de músculos y de articulaciones. Estos síntomas suelen desaparecer en un par de horas o días.
Consulte a su enfermero o médico si cualquier efecto llega a ser molesto o dura más de un par de días
- aumento de la temperatura corporal
- dolor de estómago y tripa, indigestión, náuseas, vómitos o diarrea (pérdidas intestinales)
- disminución de los niveles de calcio o fósforo en sangre
- alteraciones en los resultados de las pruebas analíticas como Gamma GT o creatinina
- un problema del ritmo cardiaco llamado ‘bloqueo de rama’
- dolor muscular o de huesos
- dolor de cabeza, sensación de mareo o debilidad
- sed, dolor de garganta, alteraciones del gusto
- hinchazón de piernas o pies
- dolor en las articulaciones, artritis, u otros problemas en las articulaciones
- problemas en la glándula paratiroidea
- cardenales
- infecciones
- un problema en sus ojos que se llama ‘cataratas’
- alteraciones en la piel
- alteraciones dentales.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- temblores o tiritona
- disminución excesiva de la temperatura corporal (‘hipotermia’)
- una enfermedad que afecta a los vasos sanguíneos del cerebro que se llama “alteración
- cerebro vascular” (infarto o hemorragia cerebral)
- alteraciones cardiovasculares (incluyendo palpitaciones, ataque al corazón, hipertensión, venas varicosas)
- alteración de las células sanguíneas (‘anemia’)
- aumento del nivel de fosfatasa alcalina en sangre
- acumulación de líquidos e hinchazón (‘linfoedema’)
- líquido en los pulmones
- problemas de estómago como ‘gastroenteritis’ o ‘gastritis’
- piedras en la vesícula biliar
- incapacidad de orinar (orina), cistitis (inflamación de la vejiga)
- migraña
- dolor en los nervios, lesión en la raíz nerviosa
- sordera
- aumento de la sensibilidad a los estímulos del sonido, del gusto, del tacto o a los cambios de olor
- dificultad al tragar
- úlceras en la boca, labios hinchados (‘quelitis’), aftas orales
- picor u hormigueo en la piel de alrededor de la boca
- dolor en la pelvis, secreción, picor o dolor vaginal
- crecimiento de la piel llamado ‘neoplasia benigna de piel’
- pérdida de memoria
- alteraciones del sueño, ansiedad, inestabilidad afectiva o cambios de humor
- erupción cutánea
- caída del cabello
- dolor o lesión en el lugar de la inyección
- pérdida de peso
- quiste en el riñón (bolsa llena de líquido en el riñón)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bondronat
- Mantener este medicamento fuera de la vista y del alcance de los niños
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica
- Tras la dilución, la solución para perfusión es estable durante 24 horas entre 2ºC y 8ºC (en nevera)
- No emplee este medicamento si observa que la solución no es transparente o que contiene partículas
6. Contenido del envase e información adicional
Composición de Bondronat
- El principio activo es ácido ibandrónico. Un vial con 6 ml de concentrado para solución para perfusión contiene 6 mg de ácido ibandrónico (como monohidrato sódico)
- Los demás componentes son: cloruro de sodio, ácido acético, acetato de sodio, agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Bondronat es una solución incolora y transparente. Bondronat se presenta en envases que contienen 1, 5 y 10 viales (vial de 6 ml de vidrio tipo I con un tapón de caucho bromobutílico). Es posible que no todos los envases estén comercializados.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Atnahs Pharma Netherlands B.V.
Strawinskylaan 3127
1077 ZX Amsterdam
Países Bajos
Responsable de la fabricación
Waymade PLC
Sovereign House,
Miles Gray Road,
Basildon, Essex,
SS14 3FR
Reino Unido
Waymade PLC
Josselin Road
Burnt Mills Industrial Estate
Basildon,
SS13 1QF
Reino Unido
Fecha de la última revisión de este prospecto: MM/AAAA
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu.
___________________________________________________________________________
La siguiente información está dirigida solamente a profesionales sanitarios
Dosificación: Prevención de Acontecimientos Óseos en Pacientes con Cáncer de Mama y
Metástasis Óseas
La dosis recomendada para la prevención de acontecimientos óseos en pacientes con cáncer de mama y metástasis óseas es de 6 mg por vía intravenosa cada 3-4 semanas. La dosis se debe perfundir durante al menos 15 minutos.
Pacientes con insuficiencia renal.
No se requiere ajuste de dosis para pacientes con insuficiencia renal leve (CLcr = 50 y < 80 ml/min).
Los pacientes con insuficiencia renal moderada (CLcr = 30 y < 50 ml/min) o con insuficiencia renal grave (CLcr < 30 ml/min), que además padecen cáncer de mama y enfermedad metastásica ósea y, que están siendo tratados para la prevención de acontecimientos óseos deben seguir las siguientes recomendaciones posológicas:
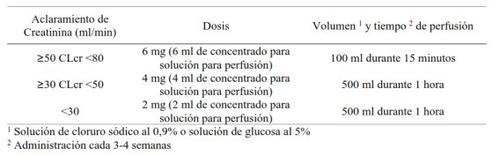
No se ha estudiado un tiempo de perfusión de 15 minutos en pacientes con cáncer con un CLcr
<50 ml/min.
Dosificación: Tratamiento de la Hipercalcemia inducida por tumores
Bondronat se administra en un entorno hospitalario. La dosis será determinada por el médico que tendrá en cuenta los siguientes factores:
Antes del tratamiento con Bondronat, el paciente debe rehidratarse adecuadamente con 9 mg/ml de cloruro sódico (al 0,9%). Debe tenerse en cuenta tanto la gravedad de la hipercalcemia como el tipo de tumor. En la mayoría de pacientes que presentan una hipercalcemia grave (calcio sérico corregido por la albúmina* ≥ 3 mmol/l o ≥ 12 mg/dl) 4 mg es una dosis única adecuada. En pacientes con hipercalcemia moderada (calcio sérico corregido por la albúmina < 3 mmol/l o <12 mg/dl) 2 mg es una dosis eficaz. La dosis máxima utilizada en los ensayos clínicos fue 6 mg, pero esta dosis no aporta ningún beneficio adicional en términos de eficacia.
- Nota: las concentraciones de calcio sérico corregido por la albúmina se calculan de la siguiente manera:
Calcio sérico corregido por la albúmina (mmol/l) Calcio sérico corregido por la albúmina (mg/dl) | = calcio sérico (mmol/l) - [0,02 x albúmina (g/l)] + 0,8 O = calcio sérico (mg/dl) + 0,8 x [4 - albúmina (g/dl)] |
Para convertir el valor del calcio sérico corregido por la albúmina de mmol/l a mg/dl, hay que
multiplicar por 4.
En la mayoría de los casos, un nivel aumentado de calcio sérico puede reducirse a niveles normales en un plazo de 7 días. La mediana del tiempo hasta la recaída (nuevo aumento por encima de 3 mmol/l del nivel sérico de calcio sérico corregido por la albúmina) fue de 18 - 19 días para las dosis de 2 mg y 4 mg. La mediana del tiempo hasta la recaída fue de 26 días con la dosis de 6 mg.
Método y vía de administración
Bondronat concentrado para solución para perfusión debe administrarse como perfusión intravenosa.
Para ello, el contenido del vial debe usarse de la siguiente manera:
- Prevención de acontecimientos óseos en pacientes con cáncer de mama y metástasis óseas- añadir a 100 ml de solución isotónica de cloruro de sodio o a 100 ml de solución de dextrosa al 5% y perfundirlo durante al menos 15 minutos. Ver la sección de dosificación arriba indicada para pacientes con insuficiencia renal.
- Tratamiento de la hipercalcemia inducida por un tumor- añadir a 500 ml de solución isotónica de cloruro de sodio o a 500 ml solución de dextrosa al 5% y perfundirlo durante 2 horas.
Nota:
Para evitar posibles incompatibilidades, Bondronat concentrado para solución para perfusión tan sólo debe mezclarse con solución isotónica de cloruro sódico o con solución de dextrosa al 5%. No debe mezclarse con Bondronat concentrado para solución para perfusión soluciones que contengan calcio.
Las soluciones diluidas son para un solo uso. Sólo se deben administrar las soluciones transparentes y sin partículas.
Se recomienda que el producto se emplee inmediatamente una vez que haya sido diluido (ver punto 5 de este prospecto: Conservación de Bondronat.
Bondronat concentrado para solución para perfusión se debe administrar mediante perfusión intravenosa. Se debe tener cuidado de no administrar Bondronat concentrado para solución para perfusión por vía intra-arterial o extravasación venosa, ya que podría producir lesiones tisulares.
Frecuencia de administración
Para el tratamiento de la hipercalcemia inducida por tumores, Bondronat concentrado para solución para perfusión se administra generalmente como una perfusión única.
Para la prevención de acontecimientos óseos en pacientes con cáncer de mama y metástasis óseas, la perfusión de Bondronat se repite en intervalos de 3-4 semanas.
Duración del tratamiento
Un número limitado de pacientes (50 pacientes) recibieron una segunda perfusión por hipercalcemia. En caso de hipercalcemia recurrente o eficacia insuficiente puede considerarse la posibilidad de repetir el tratamiento.
Para pacientes con cáncer de mama y metástasis óseas, las perfusiones de Bondronat se deben administrar cada 3-4 semanas. En los ensayos clínicos, el tratamiento se mantuvo hasta 96 semanas.
Sobredosis:
Hasta el momento no hay experiencia de intoxicación aguda con Bondronat concentrado para solución para perfusión. Teniendo en cuenta que en los estudios preclínicos a dosis altas se observó que tanto el riñón como el hígado son órganos diana en cuanto a la toxicidad, se deben controlar la función renal y hepática.
Una hipocalcemia clínicamente relevante (niveles muy bajos de calcio sérico) debe corregirse
mediante la administración intravenosa de gluconato cálcico.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BONDRONAT 6 mg CONCENTRADO PARA SOLUCION PARA PERFUSIONForma farmacéutica: COMPRIMIDO, 150 mgPrincipio activo: Ibandronico acidoFabricante: Especialidades Farmaceuticas Centrum S.A.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 2 mgPrincipio activo: Ibandronico acidoFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 6 mgPrincipio activo: Ibandronico acidoFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para BONDRONAT 6 mg CONCENTRADO PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BONDRONAT 6 mg CONCENTRADO PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes












