
AVAMYS 27.5 micrograms/spray, nasal spray suspension

How to use AVAMYS 27.5 micrograms/spray, nasal spray suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Avamys 27.5micrograms peractuation, nasalspray suspension
fluticasone furoate
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Avamys and what is it used for
- What you need to know before using Avamys
- How to use Avamys
- Possible side effects
- Storage of Avamys
- Package contents and additional information
Step-by-step guide to using the nasal spray
1. What is Avamys and what is it used for
Avamys (fluticasone furoate) belongs to a group of medicines called glucocorticoids. Avamys works by reducing inflammation caused by allergies (rhinitis), and thus reduces allergy symptoms.
Avamys nasal spray suspension is used to treat symptoms of allergic rhinitis, including nasal congestion, increased nasal secretion, and itching of the nose, sneezing, and runny or itchy eyes in adults and children 6 years and older.
Allergic symptoms may occur at certain times of the year due to allergy to plant or tree pollen (hay fever), or they may occur throughout the year due to allergy to animals, dust mites, or molds, among others.
2. What you need to know before using Avamys
Do not use Avamys
- If you are allergicto fluticasone furoate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Children and adolescents
Do not use in children under 6 years of age.
The use of Avamys:
- may cause a delay in the growth of children when used for prolonged periods. Your doctor will regularly check the child's height and ensure that the child receives the minimum effective dose.
- may cause eye disorders such as glaucoma (increased eye pressure) or cataracts (loss of lens transparency). Inform your doctor if you have had any of these disorders in the past or if you experience blurred vision or other visual disturbances while using Avamys.
Using Avamys with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
It is especially important to inform your doctor if you are using or have recently used any of the following medicines:
- corticosteroids in tablets or injectable corticosteroids
- corticosteroids in creams
- medicines for asthma
- ritonavir or cobicistat, used to treat HIV/AIDS
- ketoconazole, used to treat fungal infections
Your doctor will assess whether you should use Avamys with these medicines. Some medicines may increase the effects of Avamys, so your doctor will monitor you closely if you are taking these medicines.
Avamys should not be used at the same time as other nasal sprays containing steroids.
Pregnancy, breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Do not use Avamys if you are pregnant, or plan to become pregnant, unless your doctor or pharmacist tells you to.
Do not use Avamys if you are breastfeeding, unless your doctor or pharmacist tells you to.
Driving and using machines
Avamys is unlikely to affect your ability to drive or use machines.
Avamys contains benzalkonium chloride
This medicine contains 8.25 micrograms of benzalkonium chloride per actuation (27.5 micrograms). Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for long-term treatment. Inform your doctor or pharmacist if you experience discomfort when using the spray.
3. How to use Avamys
Follow the instructions for administering this medicine exactly as indicated by your doctor or pharmacist. Do not exceed the recommended dose. If in doubt, consult your doctor or pharmacist again.
When to use Avamys
- Use once a day.
- Use at the same time each day.
This medicine will treat your symptoms throughout the day and night.
How long it takes Avamys to work
Some people may not feel the full effects until several days after starting to use Avamys.
However, it is usually effective between 8 and 24 hours after use.
How much to use
Adults and children 12 years and older
- The usual starting doseis 2 sprays in each nostril once a day.
- Once symptoms are controlled, it may be possible to reduce the dose to 1 spray in each nostril once a day.
Children 6 to 11 years
- The usual starting doseis 1 spray in each nostril once a day.
- If symptoms are very severe, your doctor may increase the dose to 2 sprays in each nostril once a day until symptoms are under control. Then, it may be possible to reduce the dose to 1 spray in each nostril once a day.
How to use the nasal spray
Avamys has almost no taste or smell. It is sprayed into the nose as a fine mist. Be careful not to spray the medicine into your eyes. If this happens, rinse your eyes with water.
There is a step-by-step guide to using the nasal spray after section 6 of this leaflet. Follow the guide carefully to get the full benefit of using Avamys.
- See thestep-by-step guide to using the nasal spray, after section 6.
If you use more Avamys than you should
Consult your doctor or pharmacist.
If you forget to use Avamys
If you forget to take a dose, do so when you remember.
If it is almost time for your next dose, wait until then. Do not use a double dose to make up for forgotten doses.
If you have any other questions about using this medicine or if you experience any discomfort when using the nasal spray, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Allergic reactions: seek medical help immediately
Allergic reactions to Avamys are rare and affect less than 1 in 1,000 people. In a small number of people, allergic reactions can become more severe and may be life-threatening if not treated. Symptoms include:
- appearance of many wheezes (wheezing), coughing, or breathing difficulties
- sudden feeling of weakness or dizziness (leading to collapse or loss of consciousness)
- swelling of the face
- skin rash or redness.
In many cases, these symptoms will be signs of less severe side effects. However, you should be aware that they can be potentially serious– so if you notice any of these symptoms:
- Contact your doctor as soon as possible.
Very common side effects(may affect more than 1 in 10 people)
- Nosebleeds (usually minor), especially if you use Avamys for more than 6 weeks continuously.
Common side effects(may affect up to 1 in 10 people)
- Nasal ulcers – which can cause irritation or discomfort in your nose. You may notice blood when you blow your nose.
- Headache
- Shortness of breath.
Uncommon side effects(may affect up to 1 in 100 people)
- Pain, burning, irritation, painful sensation, or dryness inside the nose.
Rare side effects(may affect up to 1 in 10,000 people)
- Small holes (perforations) in the nasal septum that separates the nostrils.
Frequency not known(frequency cannot be estimated from available data)
- Slowed growth in children.
- Blurred vision or temporary changes in vision with prolonged use
- Chest tightness causing difficulty breathing.
- Voice disorder, loss of voice.
- Taste disorder, loss of taste, loss of smell.
The use of nasal corticosteroids may affect the normal production of hormones in your body, especially if used in high doses for prolonged periods. In children, these side effects may cause slower growth.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Avamys
Keep this medicine out of sight and reach of children.
It is recommended to store the Avamys nasal spray in an upright position. Always keep the cap on.
Do not use this medicine after the expiration date stated on the packaging. The expiration date is the last day of the month indicated. Once opened, the validity period of Avamys nasal spray suspension is 2 months.
Do not refrigerate or freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Avamys
- The active ingredient is fluticasone furoate. Each spray releases 27.5 micrograms of fluticasone furoate.
- The other components are anhydrous glucose, dispersible cellulose, polysorbate 80, benzalkonium chloride, disodium edetate, and purified water (see section 2).
Appearance of the Product and Container Contents
The medication is a white suspension for nasal spray contained in an amber glass bottle with a spray pump. The bottle is protected by a white plastic cover with a light blue cap and a lateral dosing button. The cover has a window to view the contents of the bottle. Avamys is available in containers of 30, 60, and 120 sprays. Only some package sizes may be marketed.
Marketing Authorization Holder
Marketing authorization:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Ireland
D24 YK11
Manufacturer
Glaxo Wellcome S.A.
Avenida de Extremadura 3
09400 Aranda de Duero
Burgos
Spain
You can request any information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tél/Tel: + 32 (0)10 85 52 00 | Lietuva GlaxoSmithKline Trading Services Limited Tel: + 370 80000334 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tél/Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 222 001 111 | Magyarország GlaxoSmithKline Trading Services Limited Tel.: + 36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: + 356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel.: + 49 (0)89 36044 8701 | Nederland GlaxoSmithKline BV Tel: + 31 (0) 33 2081100 |
Eesti GlaxoSmithKline Trading Services Limited Tel: + 372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλáδα GlaxoSmithKline Μονοπρóσωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél.: + 33 (0)1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Hrvatska GlaxoSmithKline Trading Services Limited Tel: + 385 800787089 | România GlaxoSmithKline Trading Services Limited Tel: + 40 800672524 |
Ireland GlaxoSmithKline Trading Services Limited Tel: + 353 (0)1 4955000 | Slovenija GlaxoSmithKline Trading Services Limited Tel: + 386 80688869 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika GlaxoSmithKline Trading Services Limited Tel: + 421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741 111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κúπρος GlaxoSmithKline Trading Services Limited Τηλ: + 357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Trading Services Limited Tel: + 371 80205045 |
Date of Last Revision of this Project:
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
STEP-BY-STEP GUIDE FOR USING THE NASAL SPRAY
Nasal Spray Appearance
The nasal spray is contained in an amber glass bottle protected by a plastic cover - see drawing a.It will contain 30, 60, or 120 sprays, depending on the package size prescribed for you.
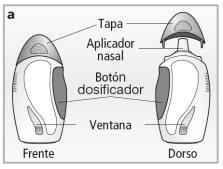
The window on the plastic cover allows you to see how much Avamys is left in the bottle. You will be able to see the liquid level in new bottles of 30 or 60 sprays, but not in a new bottle of 120 sprays because the liquid level is above the window.
Six Important Things You Need to Know About Using the Nasal Spray
- Avamys is contained in an amber glass bottle. If you need to check how much is left, hold the nasal spray upright and against a light source.This way, you will be able to see the level through the window.
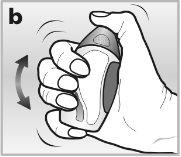
- When using the nasal spray for the first time, you will need to shake it vigorouslywith the cap on for 10 seconds. This is important because Avamys is a dense suspension that becomes liquid when shaken well - see drawing b.It will only spray when it becomes liquid.
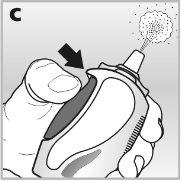
- You must pressthe dosing button firmly and completelyto release the mist through the nasal applicator - see drawing c.
- If you have difficulty pressing the button with your thumb, you can use both hands - see drawing d.
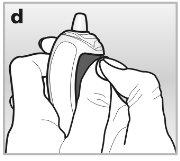
- Always keep the cap on the nasal spraywhen not in use. The cap prevents dust from entering, seals tightly under pressure, and prevents the nasal applicator from clogging. With the cap on, the dosing button cannot be pressed accidentally.
- Never use a pinor any other sharp object to unclog the nasal applicator. This would damage the nasal spray.
Preparing the Nasal Spray for Use
You must prepare the nasal spray:
- before using it for the first time
- if you have left it uncapped for 5 days or have not used the intranasal device for 30 days or more.
Preparing the nasal spray helps ensure that you always receive the full dose of medication. Follow these steps:
1Shake the nasal spray vigorouslywith the cap on for 10 seconds.
2Remove the cap by pressing firmly on the sides of the cap with your thumb and index finger - see drawing e.
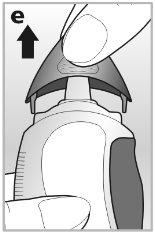
3Hold the nasal spray upright, then tilt and point the nasal applicator away from you.
4Press the dosing button firmlyand completely. Do this at least 6 timesuntil a fine spray mist is released into the air - see drawing f.
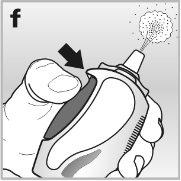
The nasal spray is now ready for use.
Using the Nasal Spray
1Shake the nasal sprayvigorously.
2Remove the cap.
3Blow your noseto clear your nostrils, then tilt your head slightly forward.
4Place the nasal applicator in one of your nostrils - see drawing g.Point the tip of the applicator slightly outward, away from the nasal septum. This helps the medication reach the right part of the nose.
5Press the dosing buttonfirmlyand completely while breathing in through your nose- see drawing h.
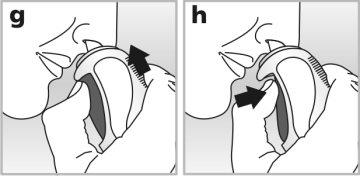
6Remove the applicator and exhale through your mouth.
7If your dose is 2 sprays in each nostril, repeat steps 4 to 6.
8Repeat steps 4 to 7 in the other nostril.
9Replace the capon the nasal spray.
Cleaning the Nasal Spray
After each use:
1Clean the applicator and the inside of the cap with a dry, clean tissue - see drawings iand j.
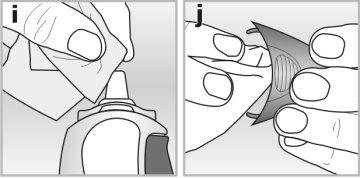
2Do not use water to clean it.
3Never use a pinor any other sharp object in the nasal applicator.
4Always replace the caponce you have finished using the nasal spray.
If the nasal spray appears not to be working:
- Check that there is still medication left. Look at the level through the window. If the level is very low, it may not be enough for the nasal spray to work.
- Check if the nasal spray has been damaged.
- If you think the nasal applicator may be clogged, do not use a pinor any other sharp object to unclog it.
- Try to restart it by following the instructions mentioned in "Preparing the nasal spray for use".
If it still does not work, or if a stream of liquid is produced, take the nasal spray to the pharmacy and consult your pharmacist.
- Country of registration
- Average pharmacy price6.99 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AVAMYS 27.5 micrograms/spray, nasal spray suspensionDosage form: NASAL PRODUCT, 27.5 MICROGRAMS/SPRAYActive substance: fluticasone furoateManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 27.5 micrograms/sprayActive substance: fluticasone furoateManufacturer: Teva B.V.Prescription requiredDosage form: NASAL PRODUCT, 27.5 micrograms/sprayActive substance: fluticasone furoateManufacturer: Cipla EuropePrescription required
Online doctors for AVAMYS 27.5 micrograms/spray, nasal spray suspension
Discuss questions about AVAMYS 27.5 micrograms/spray, nasal spray suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












