AMOROLFINE VIATRIS 50 mg/ml NAIL LACQUER

How to use AMOROLFINE VIATRIS 50 mg/ml NAIL LACQUER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Amorolfina Viatris 50 mg/ml Medicinal Nail Varnish
amorolfina
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Amorolfina Viatris and what is it used for
- What you need to know before using Amorolfina Viatris
- How to use Amorolfina Viatris
- Possible side effects
- Storage of Amorolfina Viatris
- Package Contents and Additional Information
1. What is Amorolfina Viatris and what is it used for
Amorolfina Viatris contains the active ingredient amorolfina (as hydrochloride), which belongs to a group of medications called antifungals. It is used to eliminate a wide variety of fungi that cause nail infections.
Amorolfina is used to treat fungal infections of the nails.
2. What you need to know before using Amorolfina Viatris
Do not use Amorolfina Viatris
- If you are allergic to amorolfina or any of the other components of this medication (listed in section 6).
Warnings and Precautions
This medication may cause allergic reactions, some of which can be severe. If this happens, stop applying the product, immediately remove it with a nail polish remover or the wipes provided in the package, and consult a doctor. The product should not be reapplied.
Seek immediate medical attention if you experience any of the following symptoms:
- Difficulty breathing
- Swelling of the face, lips, tongue, or throat
- Severe skin rash
Consult your doctor or pharmacist before starting to use amorolfina
- The nail varnish should not be applied to the skin surrounding the nail.
- Avoid contact of the varnish with the eyes, ears, and mucous membranes (e.g., mouth or nostrils).
- Do not inhale it.
- Wear impermeable gloves (resistant to water) when using organic solvents, such as paint thinners or turpentine, to prevent removal of the nail varnish.
Children and Adolescents
Due to the lack of available clinical experience to date, children should not be treated with amorolfina.
.
Using Amorolfina Viatris with Other Medications
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Using Other Nail Products
Cosmetic nail varnish can be used, but you should wait at least 10 minutes after applying amorolfina before painting your nails.
The nail varnish should be carefully removed before reapplying amorolfina.
Amorolfina Viatris Contains Ethanol
This medication contains 482.3 mg of alcohol (ethanol) per mL. It may cause a burning sensation on damaged skin. Do not use it near an open flame, a lit cigarette, or some devices (e.g., hair dryers), as it is flammable.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. Your doctor will decide whether you can use amorolfina.
3. How to Use Amorolfina Viatris
Follow the instructions for administration of this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Always use this medication exactly as described in this package leaflet or as your doctor or pharmacist has told you. Consult your doctor or pharmacist if you are unsure.
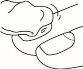
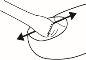
Amorolfina should be applied to the affected nails of the hands or feet once or twice a week, exactly as instructed by your doctor.
Instructions for Use
|
|
|
|
|
|
| |
|
|
|
|
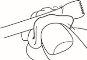
Do not use the nail files for affected nails on healthy nails, as this could spread the infection. To prevent the infection from spreading, ensure that no one else uses the files from your kit.
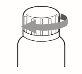
The provided spatulas are reusable. However, it is essential to wash them thoroughly with the same wipe you used to clean your nails, once you have finished the treatment steps. Avoid touching the newly treated nails with the cleaning wipe. Close the nail varnish bottle perfectly. Dispose of the wipe carefully, as it is flammable.
- Before reusing amorolfina, first remove any previous nail varnish or nail polish from your nails using a cleaning wipe, and then file your nails if necessary.
- Reapply the varnish as described above.
- Once dry, the varnish is not affected by soap and water, so you can wash your hands and feet normally. If you need to use chemical products such as paint thinners or turpentine, you should wear impermeable gloves (resistant to water) to protect the nail varnish.
- It is essential that you continue using amorolfina until the infection has disappeared and healthy nails have grown back. The duration of treatment is usually 6 months for fingernails and 9 to 12 months for toenails.
Your doctor will probably check how your treatment is progressing every 3 months.
If Amorolfina Viatris Comes into Contact with Your Eyes or Ears
If amorolfina comes into contact with your eyes or ears, rinse them immediately with water and contact your doctor or pharmacist, or go to the nearest hospital.
If You Accidentally Ingest Amorolfina Viatris
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If You Forget to Use Amorolfina Viatris
Do not worry if you forget to use amorolfina at the scheduled time. When you remember, start using the product again, exactly as before.
If You Interrupt Treatment with Amorolfina Viatris
Do not interrupt treatment with amorolfina before your doctor tells you to, as the infection may recur.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Contact your doctor immediately if you develop any of the following side effects:
Frequency not known (cannot be estimated from available data)
- Severe allergic reaction with symptoms such as dizziness or fainting, skin rash, itching, and swelling of the face, lips, tongue, or throat that can cause difficulty breathing or swallowing.
Other Possible Side Effects:
Rare (may affect up to 1 in 1,000 people):
Discolored, broken, or brittle nails.
Very Rare (may affect up to 1 in 10,000 people):
Burning sensation around the nail.
Frequency Not Known (cannot be estimated from available data):
- Redness of the skin (erythema).
- Itching of the skin (pruritus).
- Allergic skin reaction (contact dermatitis).
- Rash (urticaria) or blisters.
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medication Monitoring System: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Amorolfina Viatris
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton after "EXP". The expiration date is the last day of the month indicated.
Protect from heat. Keep the bottle tightly closed after use.
Medications should not be disposed of through wastewater or household waste. Dispose of the packaging and any unused medication in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Amorolfina Viatris
- The active ingredient is amorolfina hydrochloride. 1 ml of Amorolfina Viatris contains 55.70 mg of amorolfina hydrochloride, equivalent to 50 mg of amorolfina.
- The other components are: anhydrous ethanol, ammonio methacrylate copolymer, ethyl acetate, butyl acetate, and triacetin.
Appearance of Amorolfina Viatris and Package Contents
Ambber glass bottle with a tamper-evident plastic cap containing a clear, colorless solution.
Package sizes:
2.5 ml, 3 ml, and 5 ml
All packages contain 30 cleaning wipes, 10 spatulas, and 30 nail files.
Not all package sizes may be marketed.
Marketing Authorization Holder
Viatris Limited
Damastown Industrial Park
Mulhuddart, Dublin 15
Dublin
Ireland
Manufacturer(s):
Farmaclair
440, avenue du Général de Gaulle,
14200 Herouville Saint Clair
France
or
Creapharm Industry
29 Rue Leon Faucher
51100 Reims
France
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Viatris Pharmaceuticals, S.L.U.
C/ General Aranaz, 86
28027 - Madrid
Spain
This medication is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Spain Amorolfina Viatris 50 mg/ml Medicinal Nail Varnish EFG
Italy Amorolfina Mylan Generics 5% Smalto medicato per unghie
Portugal Amorolfina Mylan
United Kingdom (Northern Ireland) Clavusimyl 5% w/v Medicated Nail Lacquer
Sweden Amorolfin Mylan 5% Medicinskt nagellack
Date of the Last Revision of this Package Leaflet:April 2023.
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es/
- Country of registration
- Average pharmacy price21.43 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMOROLFINE VIATRIS 50 mg/ml NAIL LACQUERDosage form: NAIL POLISH, 50 mg/mlActive substance: amorolfineManufacturer: Bluefish Pharmaceuticals Ab (Publ)Prescription requiredDosage form: NAIL POLISH, 5% w/w Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Isdin S.A.Prescription requiredDosage form: NAIL POLISH, 5% w/w Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for AMOROLFINE VIATRIS 50 mg/ml NAIL LACQUER
Discuss questions about AMOROLFINE VIATRIS 50 mg/ml NAIL LACQUER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions