
Amorolak

Ask a doctor about a prescription for Amorolak

How to use Amorolak
Leaflet attached to the packaging: information for the user
Amorolak, 50 mg/ml, medicinal nail lacquer
Amorolfine
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, ask your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 3 months there is no improvement or you feel worse, you should contact your doctor.
Table of contents of the leaflet
- 1. What is Amorolak and what is it used for
- 2. Important information before using Amorolak
- 3. How to use Amorolak
- 4. Possible side effects
- 5. How to store Amorolak
- 6. Contents of the pack and other information
1. What is Amorolak and what is it used for
Amorolak is a medicine with a broad spectrum of antifungal activity, used in the local treatment of fungal infections (onychomycosis) of the nails [without matrix involvement (where the nail is formed), e.g. superficial white onychomycosis, onychomycosis under the nail, when the nail infection affects less than 50% of its surface and occurs on less than three nails]. Amorolak contains the active substance amorolfine, which has antifungal activity and kills various types of fungi that can cause nail infections.
2. Important information before using Amorolak
When not to use Amorolak
If you are allergic to the active substance - amorolfine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Amorolak, discuss it with your doctor or pharmacist.
Nail files used during treatment should not be used to care for healthy nails.
Amorolak should not be applied to the skin around the infected nail.
Avoid any contact of the medicine with the eyes, ears, and mucous membranes.
Avoid using artificial nails during treatment.
Patient who have contact with organic solvents (diluents, naphtha, etc.) during work should wear impermeable gloves to protect the Amorolak layer.
Otherwise, the Amorolak lacquer will be removed from the nail surface.
Like all medicines, this medicine can cause allergic reactions, usually mild, but some can be severe.
In such a case, you should stop using the lacquer immediately, remove the lacquer using a nail polish remover or cotton swabs provided in the packaging, and contact your doctor.
You should not reapply the lacquer.
You should contact your doctor or the nearest hospital immediately if you experience any of the following symptoms:
- breathing difficulties
- swelling of the face, lips, tongue, or throat
- severe rash.
There is no experience with the use of the medicine in patients with inflammatory changes around the nail, diabetes, circulatory disorders, malnutrition, or alcohol abuse.
Children and adolescents
There is no experience with the use of the medicine in infants, children, and adolescents.
Therefore, Amorolak should not be used in patients under 18 years of age.
Amorolak and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about medicines you plan to take.
No interactions of Amorolak with other medicines are known.
Amorolak can be used while taking other medicines.
Information on the concomitant use of the medicine with cosmetic nail polish can be found in section 3.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before using this medicine.
Since the systemic exposure to amorolfine after local application of Amorolak is negligible, no effect on pregnancy and the newborn or breastfed infant is expected.
Driving and using machines
Amorolak has no influence or insignificant influence on the ability to drive and use machines.
Amorolak contains ethanol
1 g of this medicine contains 0.552 g of alcohol (ethanol), which corresponds to 55.2% w/w.
Amorolak contains ethanol and may cause burning of damaged skin if it accidentally comes into contact with the skin around the nail.
Amorolak is flammable.
It contains ethanol, which is a flammable substance and should not be used near an open flame, lit cigarette, or certain devices, e.g. hair dryers.
3. How to use Amorolak
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist.
In case of doubt, consult your doctor or pharmacist.
Unless otherwise directed by the doctor, Amorolak should be applied as described below.
Method of administration:
For use on nails.
Amorolak is applied to infected nails of the hands and feet once a week.
Treatment should be continued until the nail plate is fully regenerated (until a healthy nail grows out).
During the application of the lacquer, the following instructions should be followed each time.
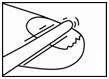
- 1. Before the firstapplication of the lacquer, as much of the diseased nail surface as possible should be filed away.
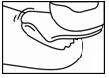
- 2. To clean the nail surface, always use one of the cotton swabs soaked in alcohol provided in the packaging.
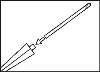
The medicinal nail lacquer should be applied using one of the provided reusable spreaders.
- 3. The lacquer should be taken up with one of the provided reusable spreaders.
- 4. Amorolak lacquer should be applied evenly to the entire diseased nail surface using the spreader.
The special shape of the spreader holder prevents the spreader from coming into contact with any surface (fungal spores remain on the spreader).
- 5. Immediately after each use, the bottle should be tightly closed to prevent the solution from drying out.
- 6. To be able to reuse the spreader, it should be cleaned with a cotton swab soaked in alcohol after use.
After applying Amorolak, a cosmetic nail polish can be applied as soon as the Amorolak layer is dry (after 10 minutes).
Before reapplying Amorolak, the cosmetic nail polish should be carefully removed.
However, you should avoid using a nail polish remover.
What else to consider during onychomycosis treatment
Nail files used during treatment should notbe used to care for healthy nails, as healthy nails could become infected.
For the treatment of diseased nails, single-use nail filesprovided in the packaging should be used.
The Amorolak layer on the fingernails may be scratched or damaged when in contact with organic solvents (cellulose thinners, turpentine derivatives, etc.).
Therefore, impermeable gloves should be worn when working with such substances to protect the Amorolak layer.
Additional important instructions during treatment
Towels should be washed as frequently as possible at a minimum temperature of 60°C.
Take care to ensure that shoes are well ventilated and dried.
Elderly patients
There are no special dosage recommendations for elderly patients.
Duration of treatment
Fungal infections are often long-lasting.
Therefore, the medicine should be used once a week continuously (as described above) until the nails are completely cured.
This usually requires treatment lasting six months (in the case of fingernails) and nine to twelve months (in the case of toenails), depending on the severity of the infection.
Nails grow out at a rate of about 1 to 2 mm per month.
Treatment evaluation is recommended at approximately 3-month intervals.
Important warning:
If the lacquer is accidentally ingested, you should contact your doctor, pharmacist, or the nearest hospital immediately.
If you feel that the effect of Amorolak is too strong or too weak, you should consult your doctor or pharmacist.
If you have any further doubts about the use of this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Possible side effects
Rare (may affect up to 1 in 1000 people):
Nail changes (e.g. change in color, brittleness, or fragility of the nails).
These symptoms may also be caused by the fungal infection itself.
Very rare (may affect up to 1 in 10,000 people):
Mild, transient burning sensation on the surface surrounding the treated nail.
Frequency not known (frequency cannot be estimated from the available data):
Severe allergic reactions have been reported, which may be associated with swelling of the face, lips, tongue, or throat, breathing difficulties, and (or) severe rash, allergic skin reaction (contact dermatitis* at the site of application or spreading beyond the application site), itching*, redness*, urticaria*, and blisters on the skin*.
*Information obtained after the product was placed on the market
Reporting of side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist.
Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Tel.: +48 22 4921301
Fax: +48 22 4921309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Amorolak
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after “EXP”.
The expiry date refers to the last day of the month.
Do not store above 30°C.
Information on the shelf life after first opening
Shelf life after first opening of the bottle: 6 months.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required.
This will help protect the environment.
6. Contents of the pack and other information
What Amorolak contains
The active substance is amorolfine.
1 ml of medicinal nail lacquer contains 55.74 mg of amorolfine hydrochloride, which corresponds to 50 mg of amorolfine.
The other ingredients are:
anhydrous ethanol, ethyl acetate, ammonio methacrylate copolymer (type A), butyl acetate, triacetin.
What Amorolak looks like and contents of the pack
Amorolak is a clear, colorless solution with a characteristic odor.
The packaging of the medicine is a brown glass bottle with a white HDPE cap, in a cardboard box.
Each packaging also contains: 10 reusable spreaders and a spreader holder as an application device, 30 nail files, and 30 cotton swabs.
Amorolak is available in packs of 3 ml or 6 ml (2 x 3 ml) of medicinal nail lacquer.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
SUN-FARM Sp. z o.o.
Dolna 21
05-092 Łomianki
tel. +48 22 350 66 69
Manufacturer
mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
SUN-FARM Sp. z o.o.
Dolna 21
05-092 Łomianki
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany: Amorocutan 50 mg/ml wirkstoffhaltiger Nagellack
Austria: Amorocutan 50 mg/ml wirkstoffhaltiger Nagellack
Poland: Amorolak
Date of last revision of the leaflet:03.2024
Instructions for nail files and alcohol-soaked cotton swabs
Distributor: SUN-FARM Sp. z o.o., 05-092 Łomianki, Poland

Follow the instructions in the patient leaflet.

Single-use nail files.

For external use only.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- Importermibe GmbH Arzneimittel Sun-Farm Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AmorolakDosage form: Varnish, 50 mg/mlActive substance: amorolfineManufacturer: Chanelle MedicalPrescription not requiredDosage form: Varnish, 50 mg/mlActive substance: amorolfineManufacturer: Laboratoires GaldermaPrescription not requiredDosage form: Varnish, 50 mg/mlActive substance: amorolfineManufacturer: Chanelle Medical Unlimited CompanyPrescription required
Alternatives to Amorolak in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Amorolak in Ukraine
Alternative to Amorolak in Spain
Online doctors for Amorolak
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Amorolak – subject to medical assessment and local rules.







