

Undofen Amorolfina

Ask a doctor about a prescription for Undofen Amorolfina

How to use Undofen Amorolfina
Leaflet attached to the packaging: patient information
Undofen Amorolfina, 50 mg/ml, medicinal nail lacquer
Amorolfine
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should tell their doctor or pharmacist. See section 4.
- If there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Undofen Amorolfina and what is it used for
- 2. Important information before using Undofen Amorolfina
- 3. How to use Undofen Amorolfina
- 4. Possible side effects
- 5. How to store Undofen Amorolfina
- 6. Contents of the packaging and other information
1. What is Undofen Amorolfina and what is it used for
- Undofen Amorolfina is an antifungal medicine in the form of a medicinal nail lacquer for topical use. The active substance of the medicine, amorolfine, acts on various types of fungi that cause nail fungus.
- The indication for using Undofen Amorolfina is nail fungus caused by dermatophytes, yeasts, and molds, and limited to two nails and the upper and lateral parts of these nails (such as in the first picture). The medicine can be used when, as a result of fungal infection of the nail plate, there is a change in the color of the nail plate (discoloration of white, yellow, or brown) or thickening of the nail plate. If the infected nail resembles those shown in the second or third picture, you should consult a doctor before using this medicine.
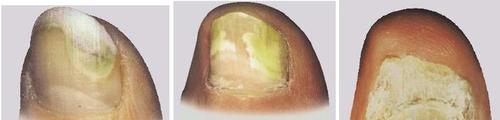
1
2
3
2. Important information before using Undofen Amorolfina
When not to use Undofen Amorolfina:
Warnings and precautions
Before starting to use Undofen Amorolfina, the patient should discuss it with their doctor or pharmacist:
The medicine is intended for application only to the nail plate. The patient should avoid contact between the nail lacquer and their eyes, ears, and mucous membranes (mouth or nose). In case of contact with the eyes or ears, they should rinse them immediately with water and contact their doctor or the nearest hospital immediately.
The medicine is flammable. Store it away from fire. Do not use it near an open flame, a lit cigarette, or certain devices (such as hair dryers).
The patient should use non-permeable protective gloves if they come into contact with solvents, as this will protect the applied medicinal lacquer from dissolving.
Children and adolescents
The medicine should not be used in children and adolescents, as there is insufficient data on its use in this age group.
Undofen Amorolfina and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
No studies have been conducted on the interaction of Undofen Amorolfina with other medicines.
Using with other nail lacquers
During treatment with the medicine, the patient should not apply cosmetics in the form of nail lacquers to the nail plate and should not use artificial nails.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine should not be used during pregnancy and breastfeeding, unless the doctor considers it absolutely necessary.
Driving and using machines
No effect of Undofen Amorolfina on the ability to drive and use machines has been demonstrated.
The medicine contains ethanol
This medicine contains 482.5 mg of alcohol (ethanol) per milliliter of lacquer, which may cause burning of damaged skin.
3. How to use Undofen Amorolfina
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
Before starting treatment
On the scheme below, the patient should mark the area of the nail affected by the disease. This information will be useful when evaluating the effectiveness of the treatment. Then, this action should be repeated every three months until the healthy nail grows back. If the fungus affects more than one nail, the scheme should show the appearance of the nail that is most affected by the disease. The patient should show the leaflet to the pharmacist or doctor if necessary to evaluate the effectiveness of the treatment.
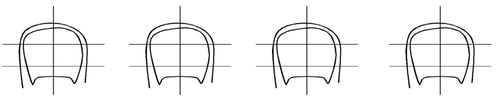
Before treatment
3 months
6 months
9 months
Dosage and method of administration
- Undofen Amorolfina medicinal nail lacquer should be applied to the surface of diseased nails on the hands or feet once a week.
- Nails grow back slowly, so it may take 2-3 months before the patient notices an improvement.
- It is essential to continue applying the lacquer until the infection disappears and the healthy nail grows back.
The patient should apply the lacquer to the nail as described below.
Step 1.Before the first application of Undofen Amorolfina, the infected nail (especially its surface) should be thoroughly filed with a nail file.
WARNING: To avoid spreading the infection, nail files used for diseased nails should not be used for healthy nails. Additionally, the patient should ensure that no one else uses these nail files.
Step 2.Then, the nail surface should be cleaned and degreased with the enclosed swab soaked in alcohol. Before reapplying the lacquer, the nail should be prepared as described above, and it should first be cleaned of any remaining lacquer using a nail file and swabs.
Step 3.The lacquer should be applied with the spatula to the entire nail surface or nails. Before applying the lacquer, the spatula should be dipped into the bottle with the lacquer and removed without wiping off the lacquer from its edge.
Step 4.After applying the lacquer, the bottle should be tightly closed as soon as possible.
Step 5.Then, the lacquer should be left to dry for 3 to 5 minutes.
WARNING:when working with organic solvents (thinners, nail polish remover, etc.), the patient should wear non-permeable gloves to protect the product from being removed from the nails.
Step 6.The spatula should be cleaned after use using the same swab that was used to clean the nail plate. The patient should avoid contact between the swab and the nail. The swab should be disposed of carefully, as it is flammable.
Treatment duration
Treatment should continue without interruption until the nail plate regenerates and the infected areas are completely cured. The treatment duration depends mainly on the severity and location of the infection. Usually, treatment lasts about 6 months for fingernail fungus and about 9 to 12 months for toenail fungus. Evaluation of treatment effectiveness is recommended at approximately 3-month intervals.


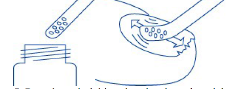

Missing a dose of Undofen Amorolfina
The patient should not worry if they forget to apply the lacquer at the right time. As soon as they remember, they should start using the lacquer again in the same way as before.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects are listed according to their frequency of occurrence:
Very common (occurring more often than in 1 in 10 people)
Common (occurring less often than in 1 in 10 people)
Uncommon (occurring less often than in 1 in 100 people)
Rare (occurring less often than in 1 in 1000 people)
Very rare (occurring less often than in 1 in 10,000 people)
Frequency not known (cannot be estimated from the available data).
Rare: nail plate disorders, nail brittleness, nail plate discoloration, excessive nail fragility with layering.
Very rare: skin burning.
Frequency not known: redness, itching, contact dermatitis, hives, skin blister.
Side effects may also be caused by the development of nail fungus.
Reporting side effects
If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181c,
02-222 Warsaw,
phone: +48 22 49 21 301,
fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will allow for the collection of more information on the safety of the medicine.
5. How to store Undofen Amorolfina
The medicine should be stored out of sight and reach of children.
Store in a temperature below 30°C. Protect from high temperature. Store the bottle in a vertical position, tightly closed.
Do not use this medicine after the expiry date stated on the carton. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Undofen Amorolfina contains
- The active substance of the medicine is amorolfine. One milliliter of lacquer contains 50 mg of amorolfine (which corresponds to 55.74 mg of amorolfine hydrochloride).
- The other ingredients are: ammonio methacrylate copolymer (type A), triacetin, butyl acetate, ethyl acetate, anhydrous ethanol.
What Undofen Amorolfina looks like and what the packaging contains
Bottle made of orange glass (type I or type III) with an HDPE cap and a tamper-evident ring, in a cardboard box.
Pack sizes: 2.5 ml, 3 ml
bottle packaged with 10 spatulas, 30 nail files, and 30 swabs.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Perrigo Poland Sp. z o.o.
ul. Domaniewska 48
02-672 Warsaw
phone: +48 (22) 852 55 51
Manufacturer
Chanelle Medical Unlimited Company
Dublin Road
Loughrea
Co. Galway
Ireland
Date of last revision of the leaflet: August 2024
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterChanelle Medical Unlimited Company
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Undofen AmorolfinaDosage form: Varnish, 50 mg/mlActive substance: amorolfineManufacturer: mibe GmbH Arzneimittel Sun-Farm Sp. z o.o.Prescription not requiredDosage form: Varnish, 50 mg/mlActive substance: amorolfineManufacturer: Chanelle MedicalPrescription not requiredDosage form: Varnish, 50 mg/mlActive substance: amorolfineManufacturer: Laboratoires GaldermaPrescription not required
Alternatives to Undofen Amorolfina in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Undofen Amorolfina in Ukraine
Alternative to Undofen Amorolfina in Spain
Online doctors for Undofen Amorolfina
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Undofen Amorolfina – subject to medical assessment and local rules.







